Method for producing tertiary butyl alcohol
A manufacturing method and technology of tert-butanol, applied in the field of manufacturing tert-butanol, can solve the problems of low hydration reaction speed, low yield, low productivity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
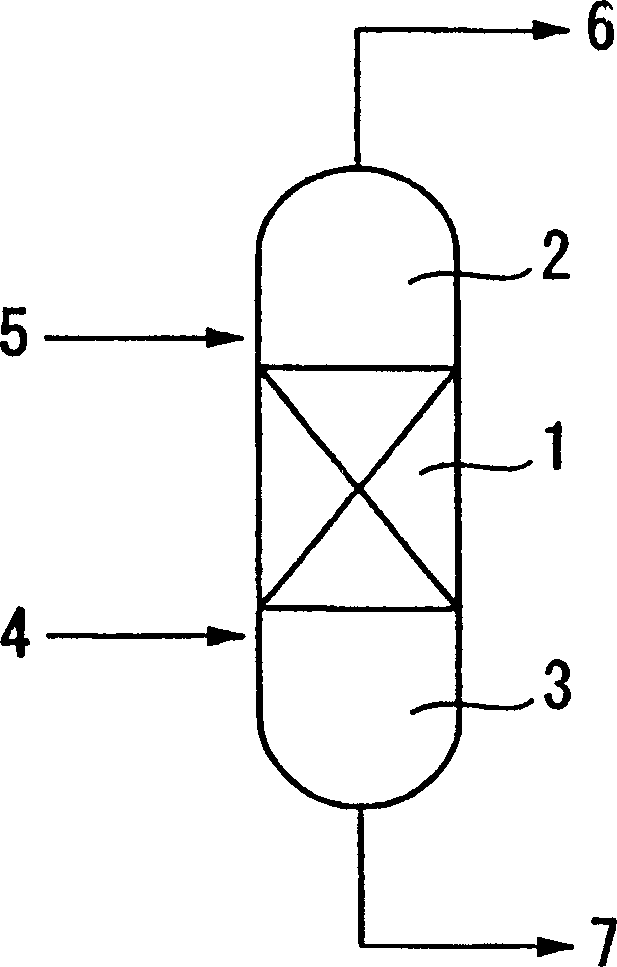
[0037]The reactive distillation apparatus is composed of a reboiler section, a reaction section, and a condenser section in order from the bottom, and these sections are respectively connected by conduits. A 1L glass autoclave (trade name "Haipa-Glasta-TEM-V" manufactured by Pressure Glass Industry Co., Ltd.) equipped with a stirrer, a thermometer, a pressure gauge, and a heating heater was used for the reboiler section, A stainless steel tubular reactor with an inner diameter of 28 mm and a length of 240 mm was used for the reaction part, and a stainless steel sleeve condenser with an inner diameter of 28 mm and a length of 200 mm was used for the condenser.
[0038] As the cation exchange resin of the catalyst, a strongly acidic microporous ion exchange resin Rebachitto Kitrist K2621 manufactured by Bayer Co., Ltd. was used. The catalyst was used by filling the reaction part with a catalyst contained in an 80-mesh wire pouch (inner diameter: 8 mm, length: 40 mm).
[0039] A...
Embodiment 2
[0048] The procedure was carried out in the same manner as in Example 1 except that the Wako Pure Chemical Industries, Ltd. product name "dimethylsulfone" was 5.2 g and the amount of water was 17.8 g. The results are shown in Table 1.
Embodiment 3
[0050] The same procedure as in Example 1 was performed except that the Wako Pure Chemical Industries, Ltd. product name "acetic acid (acetic acid)" was 6.4 g and the amount of water was 13.3 g. The results are shown in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| voidage | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com