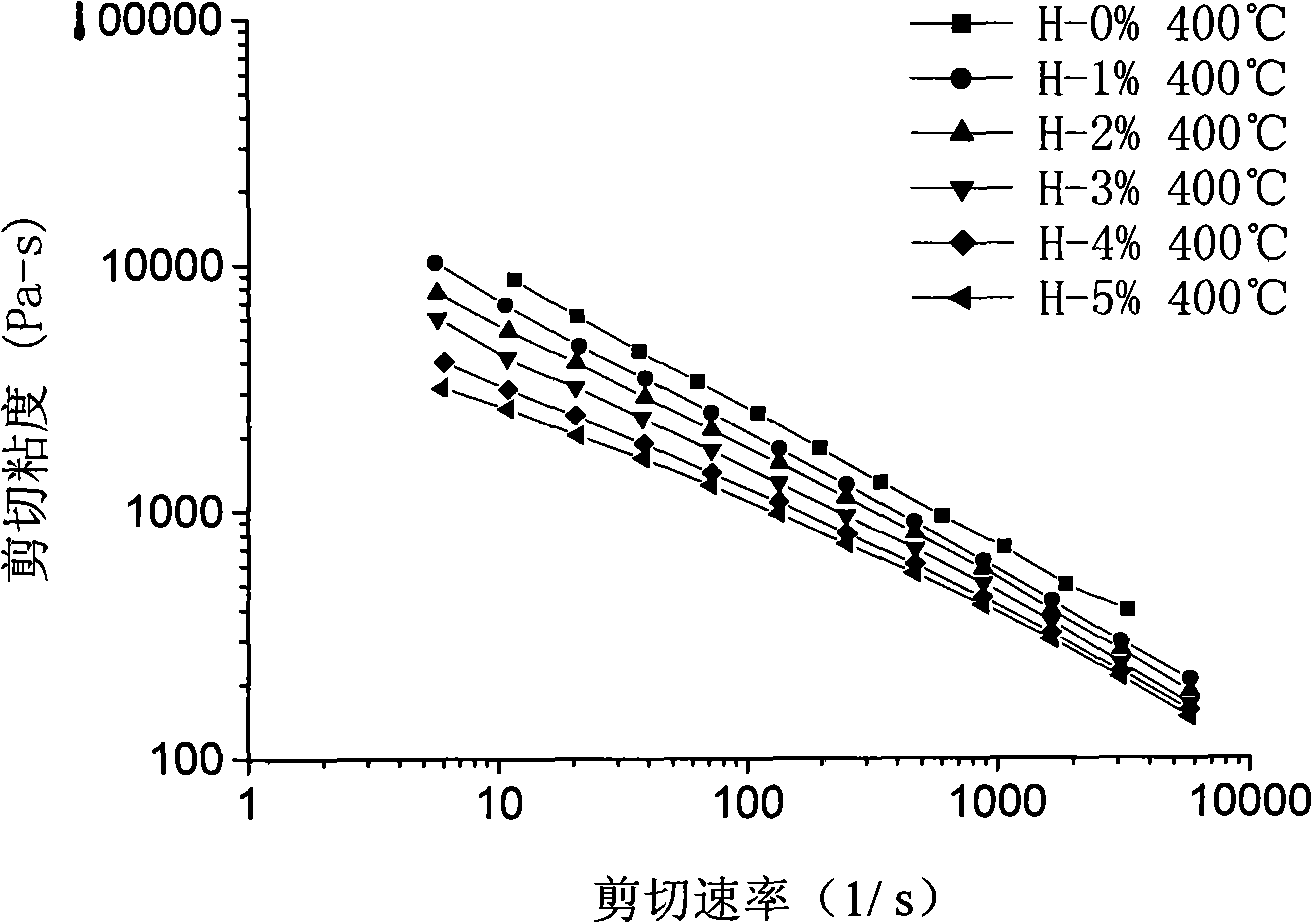
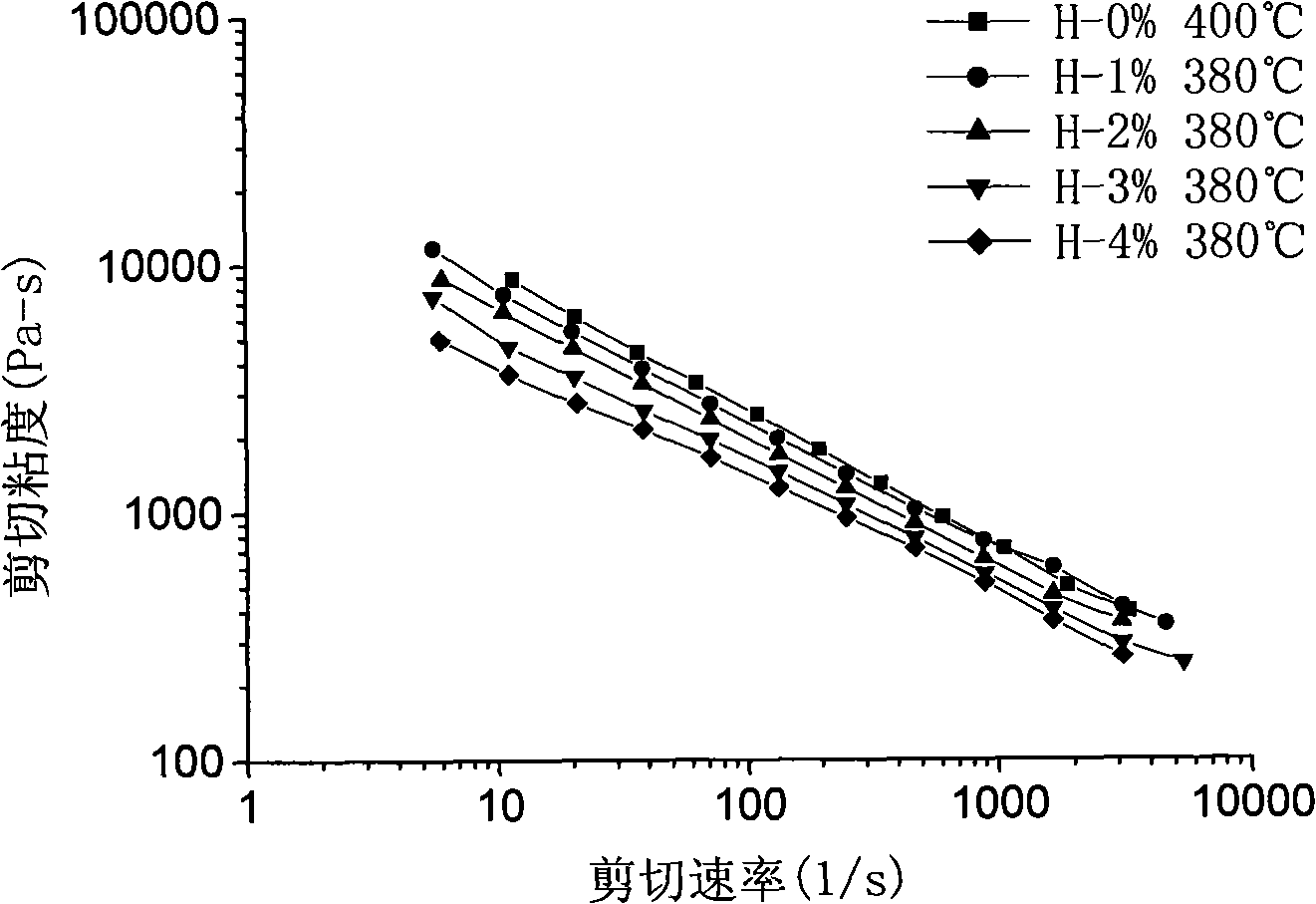
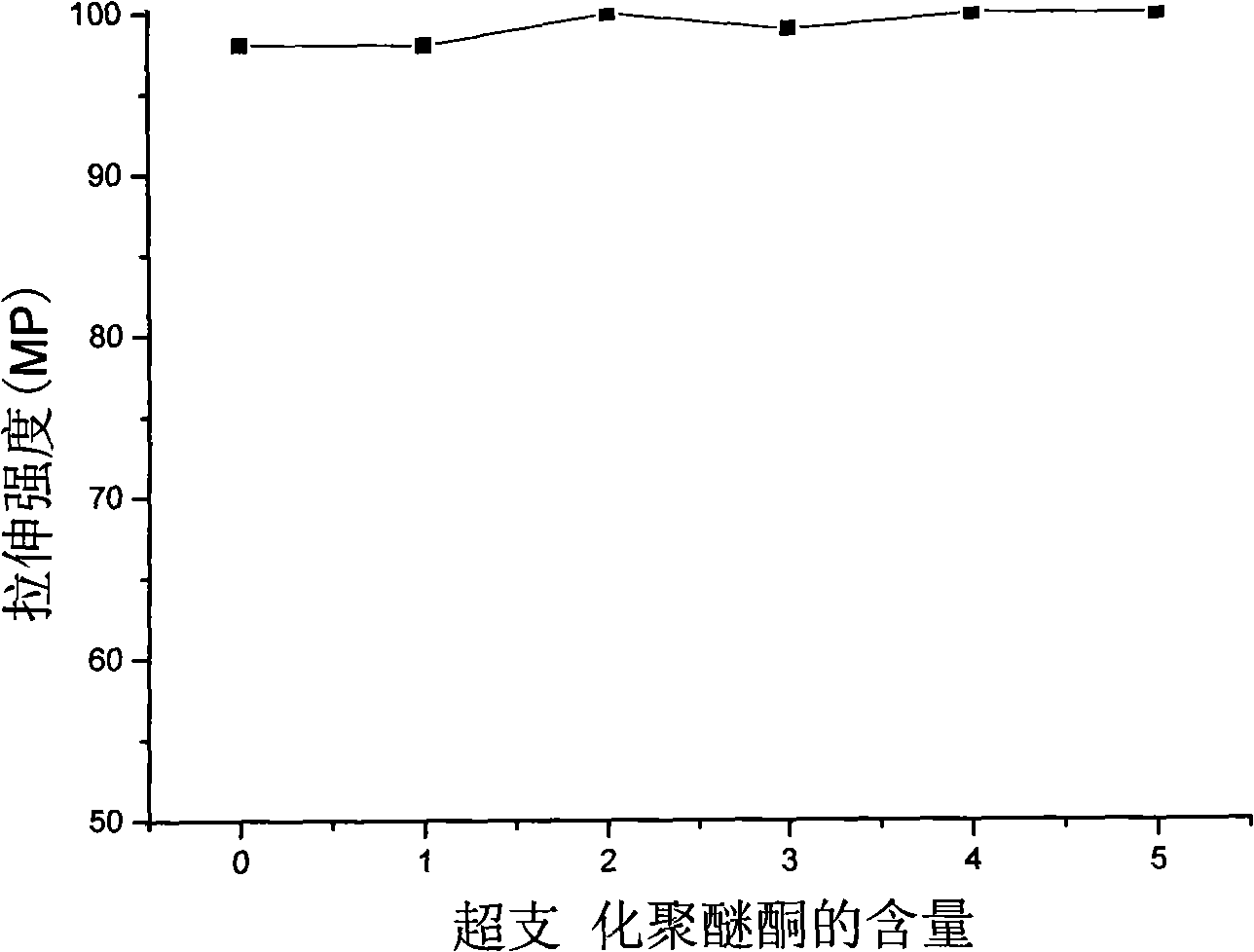
Hyperbranched poly(aryl ether ketone), preparation method and application thereof in viscosity modifier
A poly(aryl ether ketone), polycondensation reaction technology, applied in the application of hyperbranched poly(aryl ether ketone) as a viscosity modifier, preparation of A2+BB2' type of hyperbranched poly(aryl ether ketone), hyperbranched poly(aryl ether ketone) It can solve the problems of cumbersome monomer synthesis process, limited industrialization, and difficulty in gelation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Embodiment 1: Synthesis of fluorine-terminated hyperbranched polyaryletherketone (HPEEK-F)
[0050] BB 2 Preparation of 'type monomer 2, 4', 6-trifluorobenzophenone: using 2,6 difluorobenzoyl chloride and fluorobenzene to prepare by using anhydrous aluminum trichloride as a catalyst through the acylation of 2,6 difluorobenzoyl chloride and fluorobenzene. 88.25g (0.5mol) of 2,6-difluorobenzoyl chloride, 80.1g (0.6mol) of anhydrous aluminum trichloride, and 600ml of fluorobenzene as a solvent in excess, refluxed at 84°C for 8h, discharged in ice hydrochloric acid water, and weighed with petroleum ether. crystallization. For the NMR characterization of the synthesized product, see Image 6 (CDCl 3 , 500Hz, ppm) δ = 7.88-7.92 (c-H, 2H) δ = 7.44-7.48 (a-H.1H) δ = 7.14-7.18 (d-H, 2H) δ = 6.90-7.03 (b-H, 2H) (Bruker 500MHz NMR) .
[0051] Will A 2 Type monomer hydroquinone 1.10g (0.01mol), BB 2 2.36g (0.01mol) of 'type monomer 2,4',6-trifluorobenzophenone, 0.483g (0.003...
Embodiment 2
[0057] Embodiment 2: Synthesis of fluorine-terminated hyperbranched polyaryletherketone (HPEEK-F)
[0058] Will A 2 Type monomer hydroquinone 1.0725g (0.00975mol), BB 2'Type monomer 2,4', 2.36g (0.01mol) of 6-trifluorobenzophenone, 0.4709g (0.00325mol) of anhydrous potassium carbonate, 0.7235g (0.0065mol) of anhydrous sodium carbonate, and 14g of sulfolane In a three-neck flask equipped with a stirring device, nitrogen gas is passed, stirred, and the temperature is raised. The salt formation temperature is controlled at 140°C for 2 hours, 170°C for 2 hours for prepolymerization, and 210°C for 5 hours. The material is discharged into deionized water, crushed, washed three times with boiling deionized water, and dried. Reflux with ethanol for 5 times, and dry to obtain 2.5 g of hyperbranched polyaryletherketone (HFPEEK-F) whose terminal group is fluorine-terminated, and the yield is about 70%.
[0059] The glass transition temperature Tg of HPEEK-F measured by DSC is 149°C, a...
Embodiment 3
[0060] Embodiment 3: Synthesis of fluorine-terminated hyperbranched polyaryletherketone (HPEEK-F)
[0061] Will A 2 Type monomer hydroquinone 0.99g (0.009mol), BB 2 'Type monomer 2,4', 2.36g (0.01mol) of 6-trifluorobenzophenone, 0.4347g (0.0030mol) of anhydrous potassium carbonate, 0.6678g (0.0060mol) of anhydrous sodium carbonate, and 14g of sulfolane In a three-neck flask equipped with a stirring device, nitrogen gas is passed, stirred, and the temperature is raised. The salt formation temperature is controlled at 140°C for 2 hours, 170°C for 2 hours for prepolymerization, and 210°C for 5 hours. The material is discharged into deionized water, crushed, washed three times with boiling deionized water, and dried. Reflux with ethanol for 5 times, and dry to obtain 2.7 g of hyperbranched polyaryletherketone (HFPEEK-F) whose terminal group is fluorine-terminated, and the yield is about 80%.
[0062] The glass transition temperature Tg of HPEEK-F measured by DSC is 134°C, and t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Glass transition temperature | aaaaa | aaaaa |
| Glass transition temperature | aaaaa | aaaaa |
| Glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com