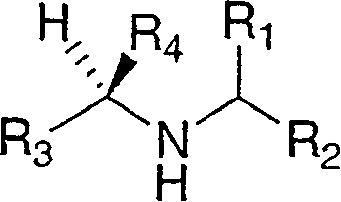
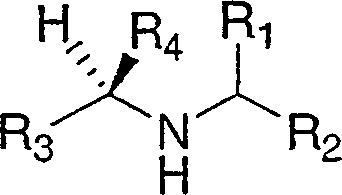
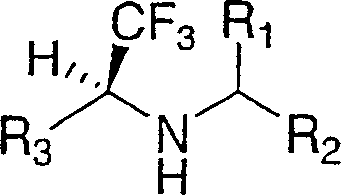
Cathepsin inhibitors
A cathepsin, compound technology, applied in anti-inflammatory agents, drug combinations, non-central analgesics, etc., can solve problems such as inactivating proteins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0249] N 1 -(cyanomethyl)-N 2 -Synthesis of -(2,2,2-trifluoro-1-phenylethyl)-L-leucine amide
[0250]
[0251] To a solution of L-leucine methyl ester hydrochloride (975 mg, 5.37 mmol) in dichloromethane (30 mL) was added 2,2,2-trifluoroacetophenone (0.75 mL, 5.34 mmol) and diisopropyl Ethylamine (3.5 mL, 20 mmol). TiCl dissolved in 0.45mL dichloromethane solution was added dropwise 4 (0.55 mL, 5.0 mmol), and the mixture was stirred overnight. Then add additional TiCl 4 (0.4 mL, 3.6 mmol), and the mixture was stirred for 3 h. Join NaCNBH 3 (1050 mg, 16.7 mmol) in methanol (20 mL), and the mixture was stirred for 2 h. Poured into 1N NaOH and extracted with ethyl acetate (2x). The organic phase was washed with 1N NaOH and brine, followed by MgSO 4 Dry and evaporate. Purification by ISCO column chromatography (30% to 90% ethyl acetate / hexanes gradient) yielded methyl N-(2,2,2-trifluoro-1-phenethyl)-L-white ester.
[0252] To a room temperature solution of methyl N-(...
Embodiment 2
[0256] N 2 -[1-(-4-Bromophenyl)-2,2,2-trifluoroethyl]-N 1 Synthesis of -(cyanomethyl)-L-leucine amide
[0257]
[0258] Using the method of Example 1, N 2 ~[1-(4-bromophenyl)-2,2,2-trifluoroethyl]-N 1 -(cyanomethyl)-L-leucine amide.
[0259] MS (-ESI): 403.9, 405.9 [M-1] -
Embodiment 3
[0261] N 1 -(cyanomethyl)-N 2 -{[4'-((methylsulfonyl))-1,1'-biphenyl-4-yl][4-((methylsulfonyl))phenyl]methyl}-L-leucine amide Synthesis
[0262]
[0263] Step 1: N-{(4-bromophenyl)[4-((methylsulfonyl))phenyl]methylene}-L-leucine methyl ester
[0264] (4-bromophenyl)[4-((methylsulfonyl))phenyl]methanone (202mg, 0.59mmol), L-leucine methyl ester hydrochloride (328mg, 2.0mmol) and camphorsulfonic acid (52 mg, 0.22 mmol in toluene was refluxed for 18 h using a Dean-Stark trap. The solvent was removed in vacuo and the residue was purified by chromatography using EtOAc and hexanes as eluents to afford the title compound along with the starting material ( A mixture of 4-bromophenyl)[4-((methylsulfonyl))phenyl]methanone in a ratio of 1:1.
[0265] Step 2: N-{(4-bromophenyl)[4-((methylsulfonyl))phenyl]methyl}-L-leucine methyl ester
[0266] To N-{(4-bromophenyl)[4-((methylsulfonyl))phenyl]methylene}leucine methyl ester and (4-bromophenyl Base)[4-((methylsulfonyl))phenyl]methan...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com