Synthesis of beta-l-2'-deoxy nucleosides
A nucleoside, a suitable technology, applied in organic chemistry, sugar derivatives, etc., can solve the problems of dioxane flammability, unsuitable industrial methods, and easy formation of peroxides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0174] In a first embodiment, 2'-deoxythymidine ( Figure 4 ). The synthesis method comprises: (a) first oxidizing D-xylose with aqueous bromine, then acetic acid and hydrobromic acid to form 2,5-dibromo-2,5-dideoxy-D-lyxenoic acid-1, 4-lactone (2); (b) reacting the lactone product of step (a) with potassium iodide in trifluoroacetic acid (TFA) to give the corresponding 5-iodo compound, i.e. selective removal of the C-2 bromine atom , to obtain 5-iodo-2-deoxylactone (3); (c) treating 5-iodo-2-deoxylactone with potassium hydroxide aqueous solution to obtain 4,5-epoxidized derivatives (4); (d ) Treat 4,5-epoxidized derivatives with aqueous acid to generate corresponding 2-deoxy-L-ribonolactone (5) through C-4 stereospecific conversion; (e) by combining with any protecting group such as Reaction of toluoyl chloride in TEA protects the C-3 and C-5 positions (6); (f) Selective reduction of the protected 2-deoxy-L-ribonolactone with Red-Al reducing agent gives the corresponding in...
Embodiment 1
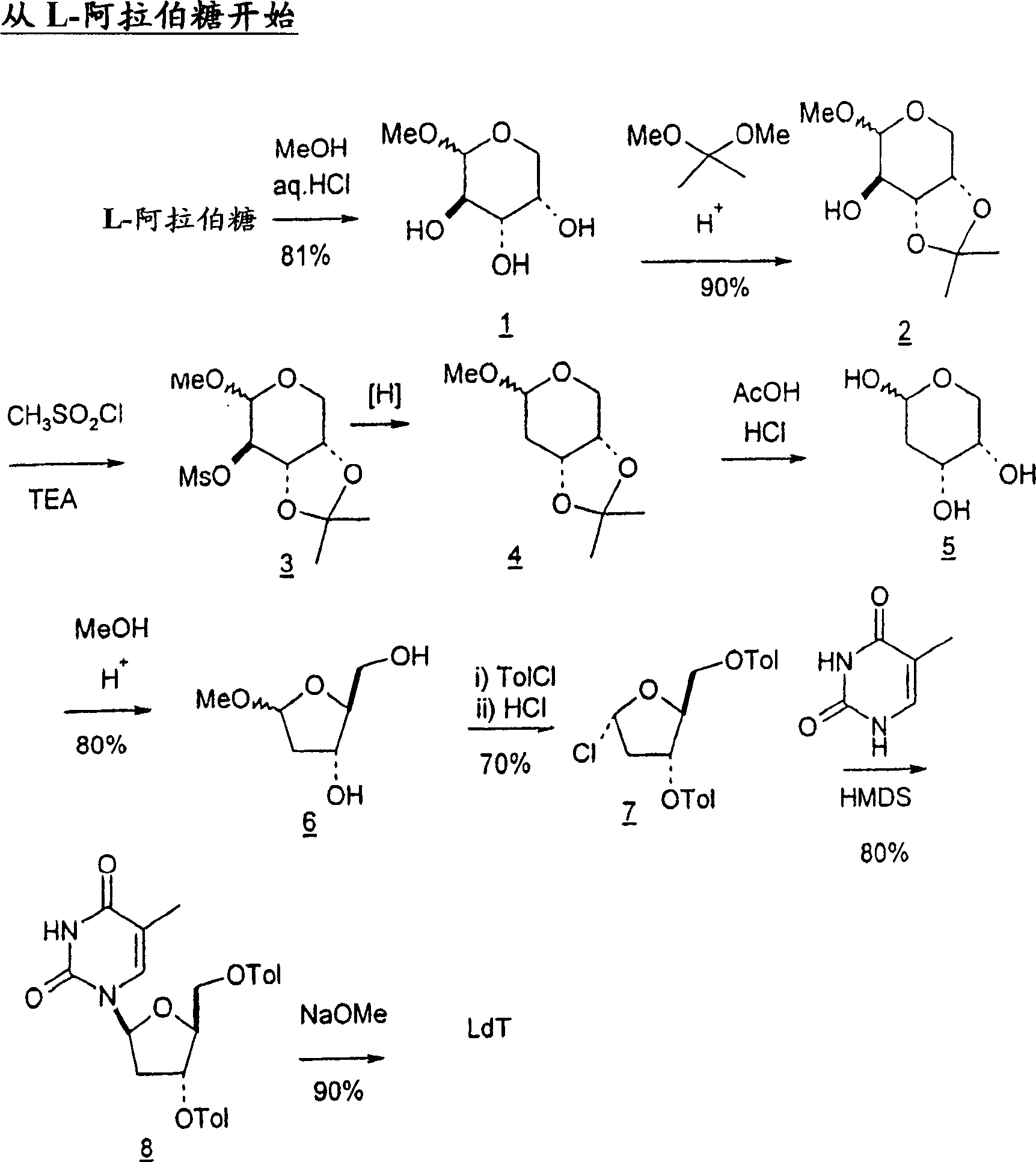
[0250] The L-arabinose is converted to the corresponding methyloside and the 3- and 4-hydroxyl groups are protected as acetonated derivatives. The following scheme shows a method for deoxidizing the 2-hydroxyl group of compound 2 by converting the hydroxyl group to the corresponding mesylate group and subjecting the mesylate intermediate to reductive cleavage conditions to give 2-deoxy intermediate 4. See H. Urata, E. Ogura, K. Shinohara, Y. Ueda and M. Akagi, Nucleic Acids Res. 1992, 20, 3325-3332; and J.W. Pratt, N.K. Richtmyer and C.S. Hudson, J.Am.Chem.Soc .1952, 74, 2200-2205.
[0251] Start with L-arabinose
[0252]
Embodiment 2
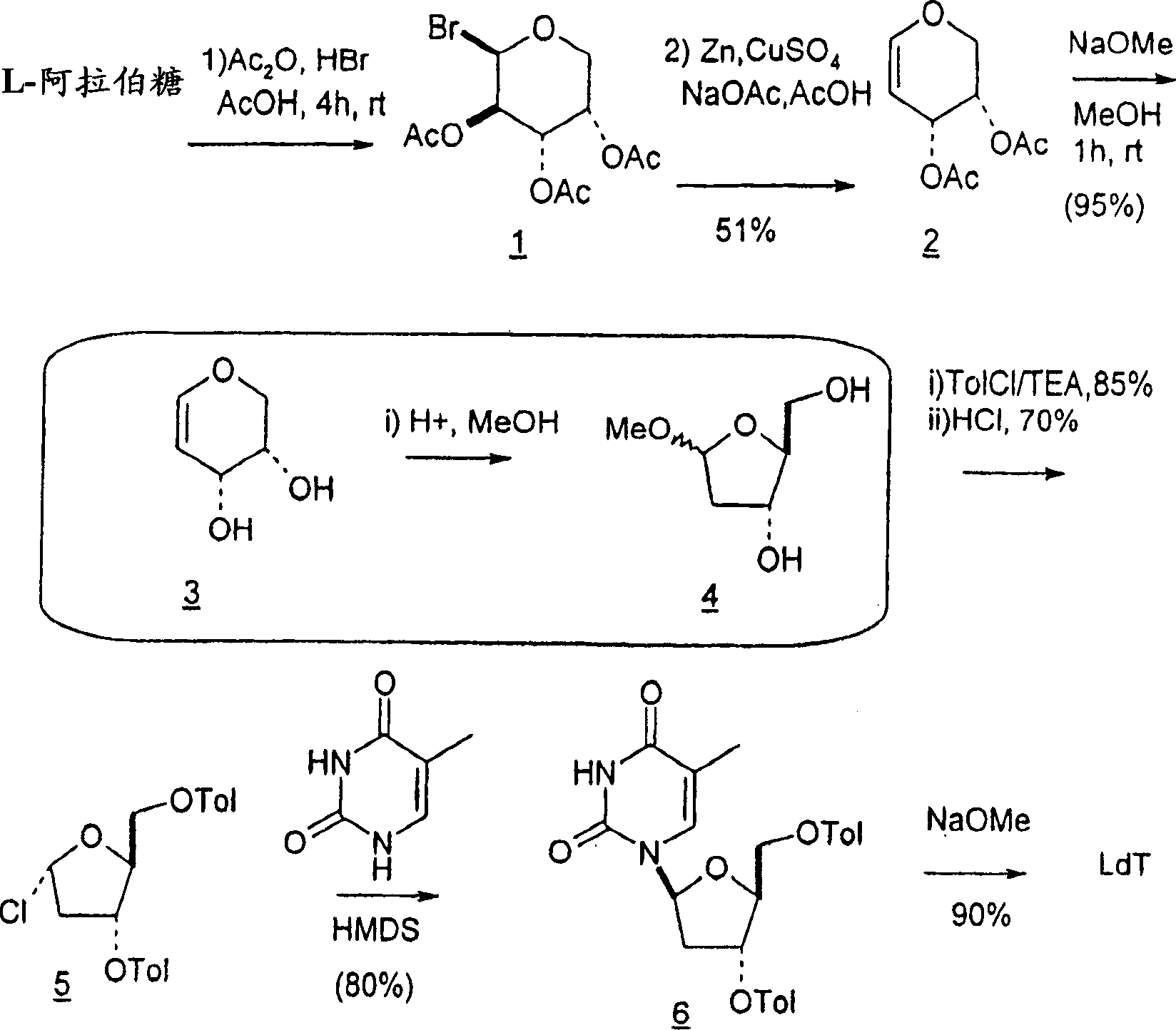
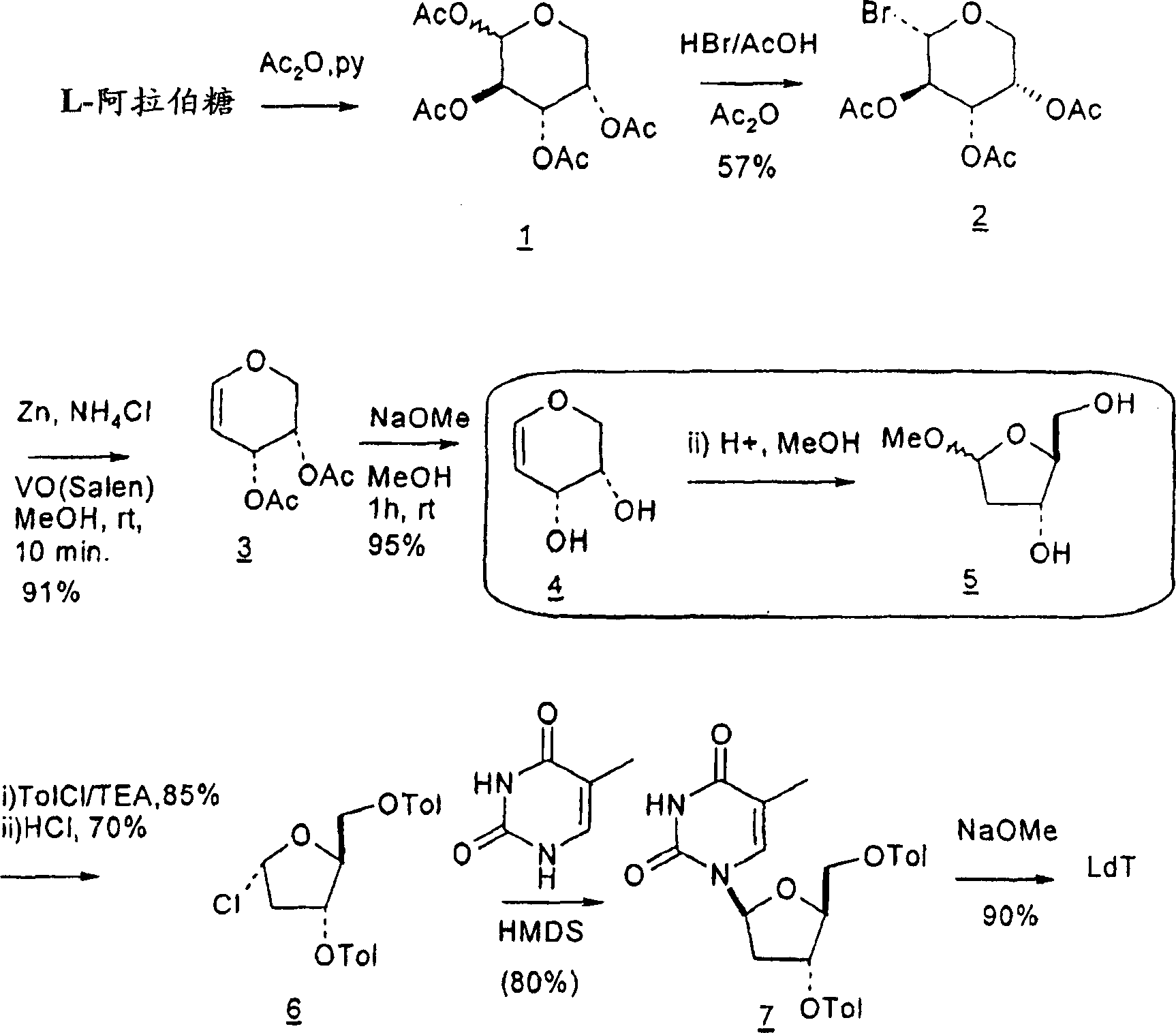
[0254]The conversion of L-arabinose to the corresponding enose derivatives and the conversion of the resulting enose intermediates to methyl 2-deoxyribofuranosides is accomplished through key reductive elimination steps. See B. K. Shull, Z. Wu and M. Koreeda, J. Carbohydr. Chem. 1996, 15, 955-964; M. L. Sznaidman, M. R. Almond and A. Pesyan. Nucleosides, Nucleotides & Nucleic Acids 2002, 21, 155-163; and Z.-X. Wang, W. Duan, L.I. Wiebe, J. Balzarini, E.D. Clercq and E.E. Knaus, Nucleosides, Nucleotides & Nucleic Acids 2001, 20, 11-40.
[0255]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com