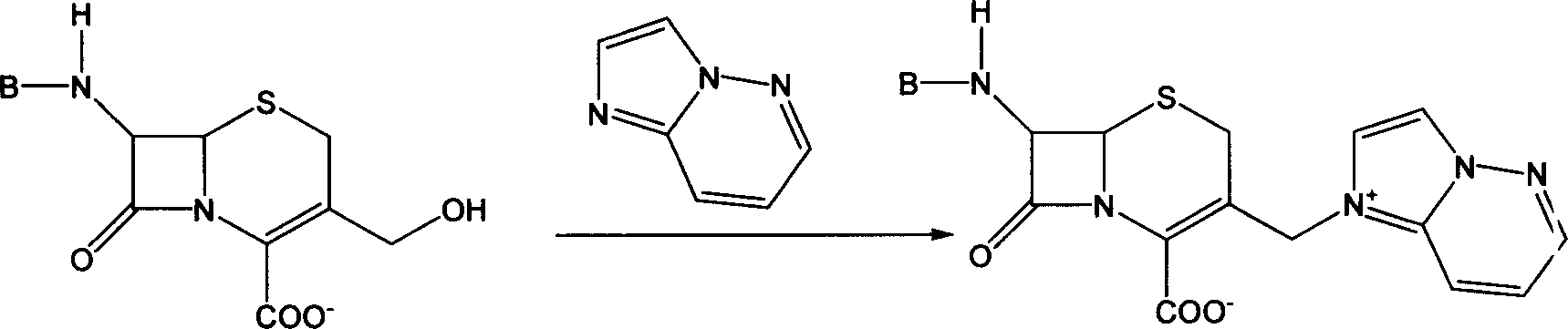
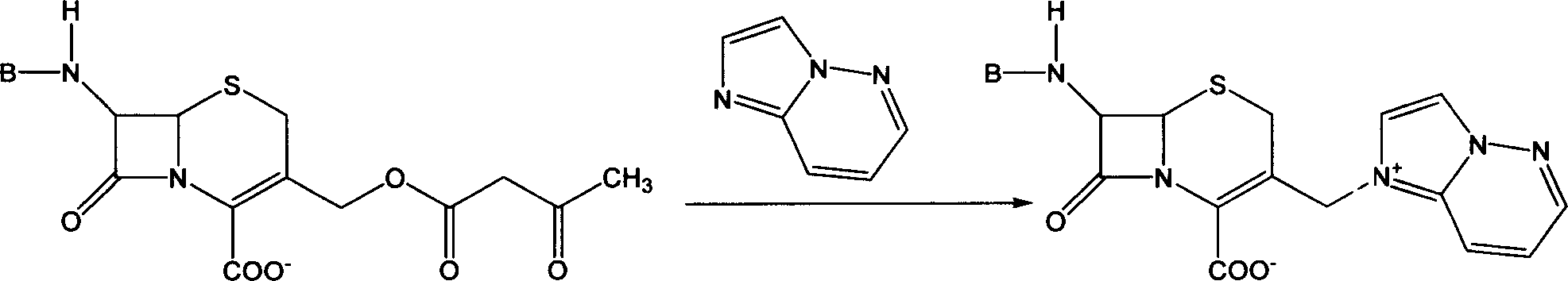
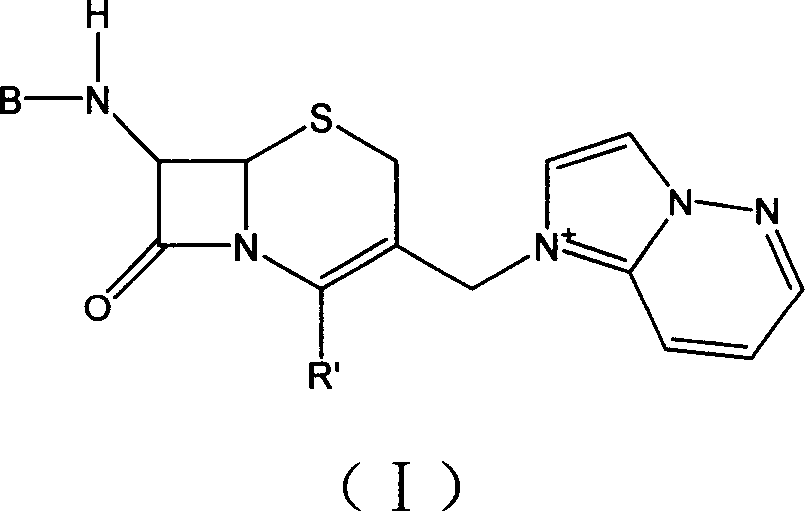
'One kettle process' of preparing cefozopran and its intermediate
A technology of compound and amino group, which is applied in the field of preparation of cefozopran and its intermediates, can solve the problems that the product cannot be prepared economically and the starting materials are not easy to obtain, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Under nitrogen flow, add 100 g (0.37 mol) of 7-aminocephalosporanic acid (7-ACA) to 500 ml of Freon F113a, stir and cool to -10°C, add 1.1 mol of triethylamine dropwise under stirring, and control the temperature at -10°C At ~-5°C, 88.9 g (0.44 mol) of iodotrimethylsilane and 52.9 g (0.44 mol) of imidazo[1,2-b]pyridazine were added, and stirred at room temperature for 4 hours. After the reaction was completed, 200 ml of 2N hydrochloric acid aqueous solution was added, and 2000 ml of isopropanol was added to precipitate crystals. Suction filtration, the filter cake was washed with ice-cold isopropanol, and vacuum-dried to obtain 3-(imidazo[1,2-b]pyridazin-1-yl)methyl-3-cephem hydrochloride as a yellow-white solid 75 grams, yield: 75%.
Embodiment 2
[0043] 50 g (0.14 mol) of 3-(imidazo[1,2-b]pyridazin-1-yl)methyl-3-cephem hydrochloride prepared according to Example Method 1 was added to 100 ml of N, -N-diformyl formamide (DMF) and 100 ml of water mixed solution, add (5-amino-1,2,4-thiadiazol-3-yl)-2-methoxyiminoacetic acid mercapto 59 g (0.168 mol) of benzothiazole active ester, adjust the pH of the reaction system to 8-10 with 1N aqueous sodium hydroxide solution, and stir for about 2 hours. Add hydrochloric acid to adjust the pH to 2-3, and add isopropanol dropwise to precipitate crystals. Suction filtration and vacuum drying gave 54.1 g of cefozopran hydrochloride, yield: 70%.
Embodiment 3
[0045] Under nitrogen flow, 150 grams (0.29mol) of 7β-[(Z)-2-(5-amino-1,2,4-thiadiazol-3-yl)-2-methoxyiminoacetyl]-cephalosporin Add alkanoic acid to 600ml Freon F113a, stir and cool to -10°C, add 0.87mol triethylamine dropwise under stirring, control temperature -10~-5°C, add 88.9g (0.35mol) iodotrimethylsilane. Stir for 3 hours, add 52.9 g (0.44 mol) of imidazo[1,2-b]pyridazine, and stir at room temperature for 5 hours. Add 400 ml of 2N hydrochloric acid aqueous solution, add 4000 ml of isopropanol, and precipitate crystals. After suction filtration, the filter cake was washed with ice-cold isopropanol, and dried in vacuum to obtain 96 g of cefozopram hydrochloride, yield: 60%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com