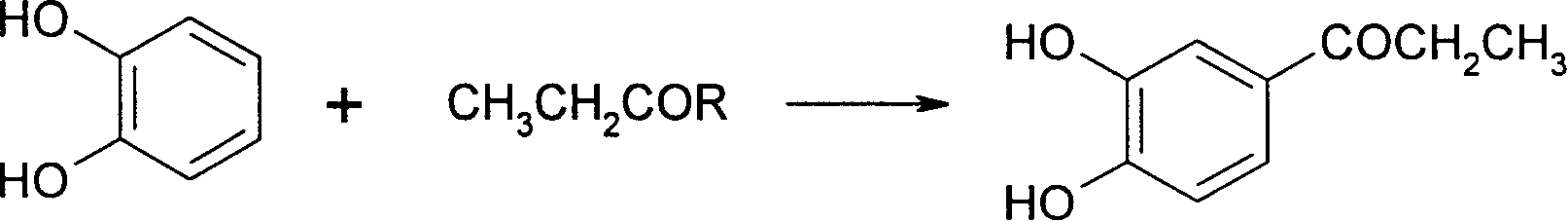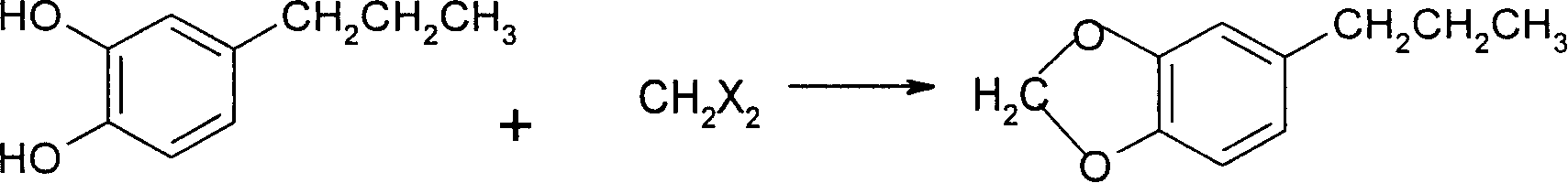Method for preparing dihydro safrole
A technology of dihydrosafrole and dihydroxybenzene, applied in the direction of organic chemistry, can solve the problems of low rearrangement yield, many reaction steps, high industrialization cost, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] (1) In the reactor with condensing reflux, stirring and dropping and heating device, add 111.3g catechol, 500ml dichloroethane, 166g ZnCl 2 , stir, cool down to 0-5°C, slowly add 140g of propionyl chloride dropwise, drop it in about 2 hours, then raise the temperature to 20°C, continue to react for 15 hours, after the reaction is completed, add water to dilute, separate the organic phase, and carry out Pickling and washing with water, drying over sodium sulfate, precipitation, and recrystallization of the residue with ethanol to obtain 144 g of solid 3,4-dihydroxypropiophenone, melting point: 145-146°C, content: 98%, yield: 85%.
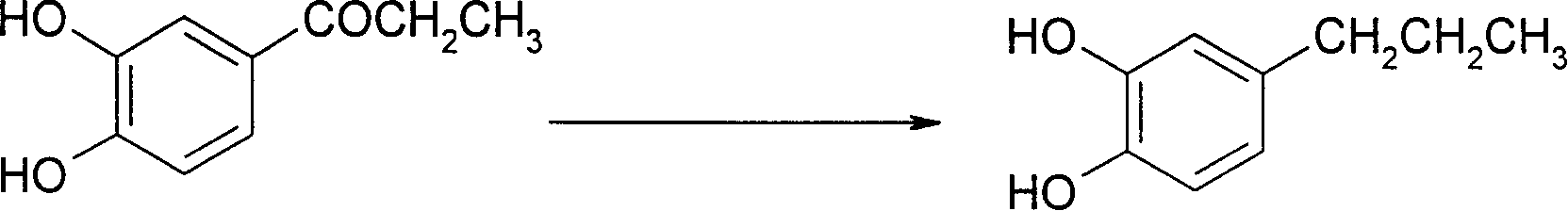
[0027] (2) 85g of solid 3,4-dihydroxypropiophenone obtained in the last step is dissolved in 300ml ethanol, and the Pd / C catalyst of 2g5% is added, then the mixture is placed in an autoclave, and under a pressure of 10atm Hydrogenation at 40-45°C, after hydrogenation for about 6 hours, the reaction is over, the mixture is filtered, the filtrat...
Embodiment 2
[0030] (1) In the reactor with condensing reflux, stirring and dropping and heating device, add 111.3g catechol, 240ml dichloroethane, 2ml70% perchloric acid, stir, cool to 10~15°C, Slowly add 144g of propionic anhydride dropwise, drop it in about 2 hours, then raise the temperature to 20°C, and continue the reaction for 15 hours. dissolved, and the residue was recrystallized from ethanol to obtain 136.5 g of solid 3,4-dihydroxypropiophenone, melting point: 145-146° C., content: 98.1%, and yield: 80.5%.
[0031] (2) 85g of solid 3,4-dihydroxypropiophenone obtained in the last step is dissolved in 900ml ethanol, and the Pd / C catalyst of 5g5% is added, then the mixture is placed in an autoclave, and under a pressure of 10atm Hydrogenation at 20-25°C, after hydrogenation for about 7 hours, the reaction is over, the mixture is filtered, the filtrate is desolvated under reduced pressure, and the residue is recrystallized with ethanol to obtain 69g of solid 4-propylcatechol, melting...
Embodiment 3
[0034] (1) In the reactor with condensing reflux, stirring and dropping and heating device, add 111.3g catechol, 240ml dichloroethane, 170g AlCl3 , stirred, cooled to 0-5°C, slowly added dropwise 280g of propionyl chloride, dripped in about 2 hours, then raised to 70°C, continued to react for 15 hours, after the reaction was completed, the post-treatment was the same as in Example 1 step (1) to obtain a solid 104g of 3,4-dihydroxypropiophenone, the content is 97%, and the yield is 61%.
[0035] (2) 85g of solid 3,4-dihydroxypropiophenone obtained in the last step is dissolved in 600ml ethanol, and the Pd / C catalyst of 2g5% is added, then the mixture is placed in an autoclave, and under a pressure of 60atm Hydrogenation at 40-45° C. After hydrogenation for about 6 hours, the reaction was completed, and the aftertreatment was the same as step (2) of Example 1 to obtain 70 g of solid 4-propylcatechol with a content of 98% and a yield of 91%.
[0036] (3) Dissolve 31 g of solid 4-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com