Method for detecting medicine material astragalus quality
A quality detection method and technology for medicinal materials, which are applied in the field of fingerprint quality detection of astragalus medicinal materials and their extracts, can solve the problems of less research on fingerprints and the like, and achieve the effect of stable quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
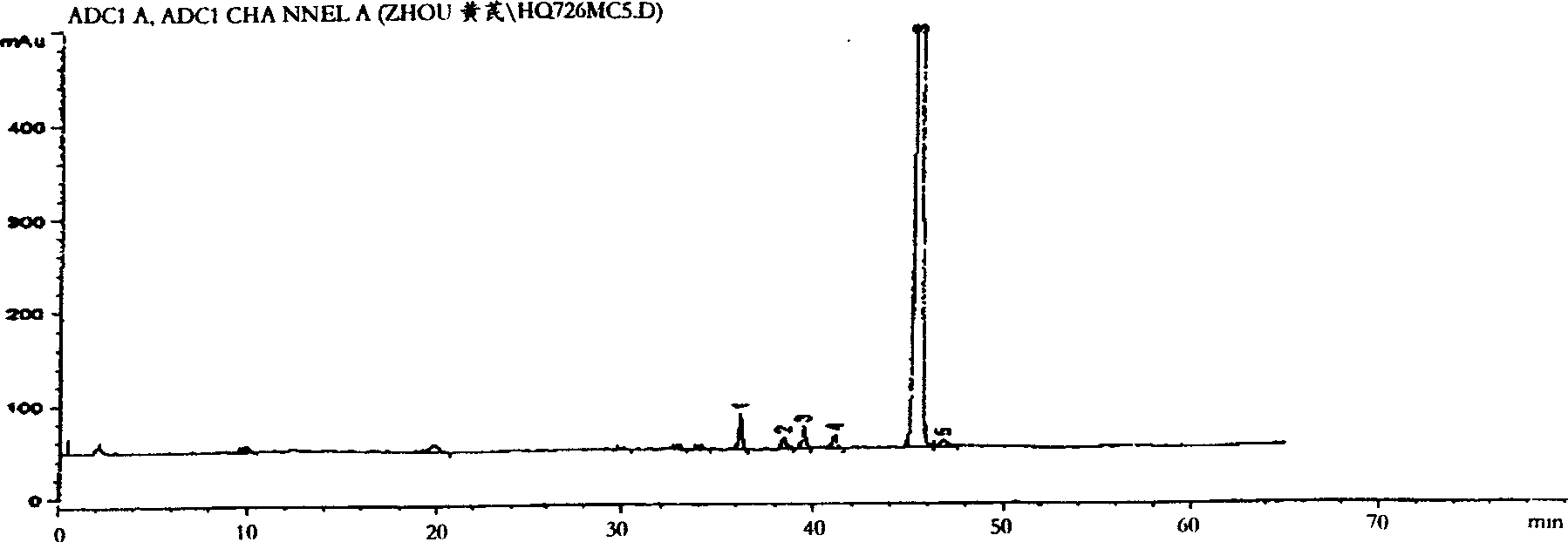
[0015] Experimental example 1 Determination of the common peaks of the fingerprint of Radix Astragali
[0016] 1. Fingerprint
[0017] According to the relevant parameters given by 10 batches of test sample HPLC chromatograms, the main chromatographic peaks of Astragalus medicinal material measured by the aforementioned preparation method all appeared within 60 minutes; the 2-hour test sample solution chromatogram showed that it did not appear after 60 minutes Chromatographic peaks. Comparing the chromatograms of each batch of samples, it was found that there were 7 peaks common to each batch, and thus a standard fingerprint was established.
[0018] 2. Calibration of common fingerprint peaks
[0019] According to the calculation results of relevant data of 10 batches of test product fingerprints, the common peak average relative retention time (peak number) is 0.387 (1), 0.802 (2), 0.852 (3), 0.875 (4), 0.909 (5) , 1.000(S), 1.030(6), as the basis for common fingerprint pe...
experiment example 2
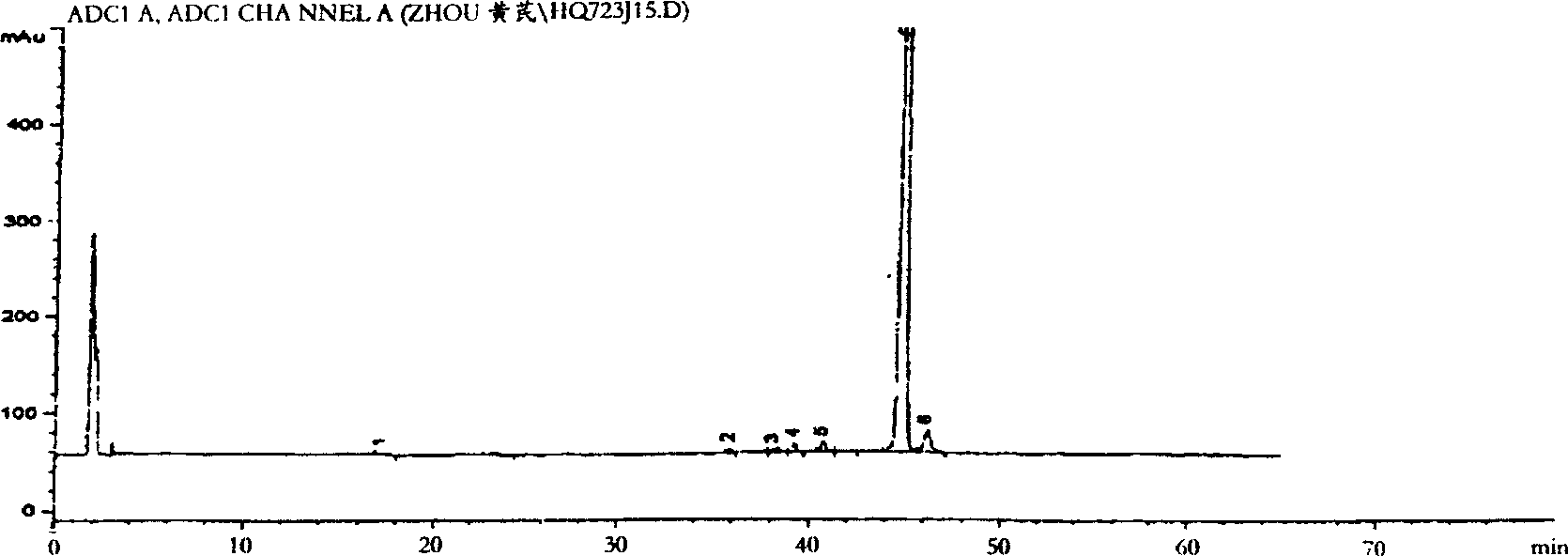
[0031] Experimental example 2 Determination of the common peaks of astragaloside fingerprint
[0032] 1. Fingerprint
[0033] According to the relevant parameters given by 3 batches of test sample HPLC chromatograms, the main chromatographic peaks of astragaloside measured by the aforementioned preparation method all appeared within 60 minutes; the chromatogram of need test solution in 2 hours showed that it did not appear after 60 minutes Chromatographic peaks. Comparing the chromatographic peaks of each batch of samples, it was found that 6 peaks were common to each batch, thus establishing a standard fingerprint.
[0034] 2. Calibration of common fingerprint peaks
[0035] According to the calculation result of relevant data of 3 batches of test product fingerprints, each common peak average relative retention time (peak number) is successively 0.797 (1), 0.847 (2), 0.870 (3), 0.905 (4), 1.000 (S ), 1.029(5), as the basis for the common fingerprint peak calibration, the ...
experiment example 3
[0047] Experimental Example 3 Astragalus test product solution stability test
[0048] Get the Astragalus membranaceus test solution of the present invention as the measurement object, measure according to the aforementioned chromatographic conditions on the day of preparation, the next day, and the fourth day (preserved at 4°C), record the retention time and peak area of each common chromatographic peak, and use the reference substance Astragalus membranaceus The retention time of glycoside is used as a reference, and the ratio of the relative retention time of each common peak is converted, and the results are shown in Tables 1-2. The results show that the relative standard deviation of the relative retention time of each common peak is less than 2%, which is very stable; the relative standard deviation of the peak area percentage of the reference peak is less than 2%. The above results show that the test solution is stable within 4 days. See Tables 7 and 8.
[0049] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com