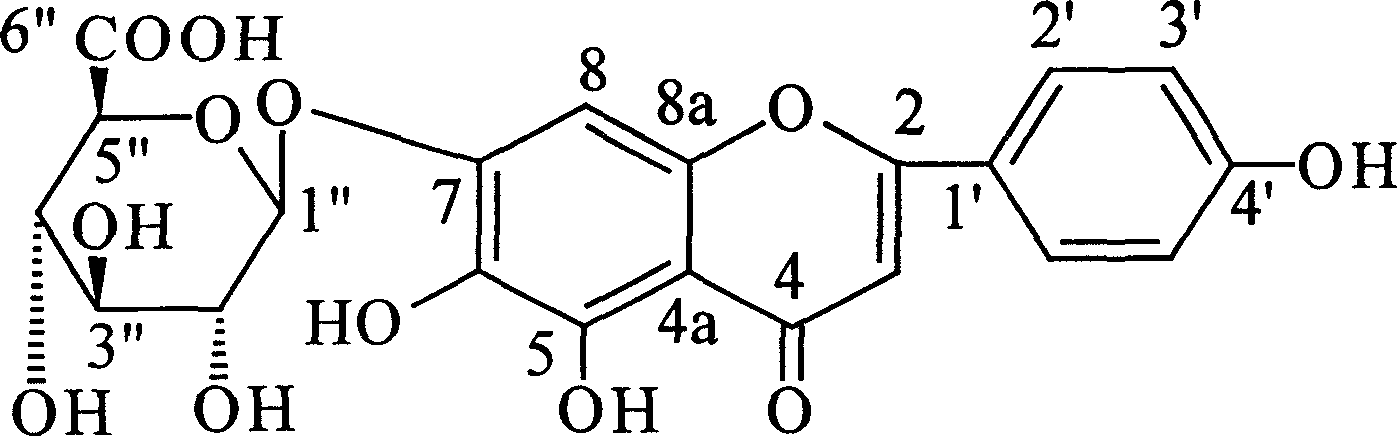
High purity wild Radix scutellariae glucoside medicine composition, and application of making medicine to treat diseases of cardiovascular and cerebrovascular
A scutellarin and high-purity technology, which is applied in the field of high-purity scutellarin pharmaceutical compositions, can solve problems such as difficulty in ensuring long-term medicinal requirements, content reduction, and the like, and achieve reliable pharmacological effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Prescription: scutellarin powder 40g
[0025] Polysorbate 80 4g
[0026] Vitamin C 20g
[0027] Sodium bicarbonate amount
[0028] Add water for injection to 20000ml
[0029] Add 40g of scutellarin powder into 10000ml of water for injection, add sodium bicarbonate solution until completely dissolved; add 4g of polysorbate 80, 20g of vitamin C and stir for 5 minutes, the main drug and auxiliary materials have been dissolved; add water for injection until The whole amount is adjusted to pH=7.05 with sodium bicarbonate solution, finely filtered, potted, and sterilized to obtain scutellarin injection. The content is 98.8%, and the pH value is 7.0; after measurement and calculation, the content is 96.3%, and the pH value is 6.80 after being stored in the dark at room temperature for one and a half years. (Nitrogen filling protection in the whole process)
Embodiment 2
[0031] Prescription: scutellarin powder 40g
[0032] EDTA-2Na 6g
[0033] Vitamin C 20g
[0034] Sodium bicarbonate amount
[0035] Add water for injection to 20000ml
[0036] Add 40g of scutellarin powder into 10000ml of water for injection, add sodium bicarbonate solution until completely dissolved; add 6g of EDTA-2Na, 20g of vitamin C and stir for 5 minutes, the main drug and auxiliary materials have been dissolved; add water for injection to the full amount , adjust pH=6.95 with sodium bicarbonate solution, fine filter, potting, and sterilize to obtain scutellarin injection. The content is 98.7%, and the pH value is 6.91. After measurement and calculation, the content is 96.5%, and the pH value is 6.87 after being stored at room temperature in the dark for one and a half years. (Nitrogen filling protection in the whole process)
Embodiment 3
[0038] Prescription: scutellarin powder 40g
[0039] Vitamin C 10g
[0040] Sodium bicarbonate amount
[0041] Add water for injection to 20000ml
[0042] Add 40g of wild scutellarin powder into 10000ml of water for injection, add sodium bicarbonate solution until completely dissolved; add 10g of vitamin C, stir for 5 minutes, the main drug and auxiliary materials have been dissolved; The sodium solution was used to adjust the pH to 7.10, finely filtered, potted, and sterilized to obtain the scutellarin injection. The content is 98.5%, and the pH value is 7.02. After measurement and calculation, the content is 96.8%, and the pH value is 6.91 after being stored at room temperature in the dark for one and a half years. (Nitrogen filling protection in the whole process)
[0043]
Sample source
Content stability
0 months
June
December
18 months
Example 1
98.8%
98.2%
97.1%
96.3%
Example 2
98.7%...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com