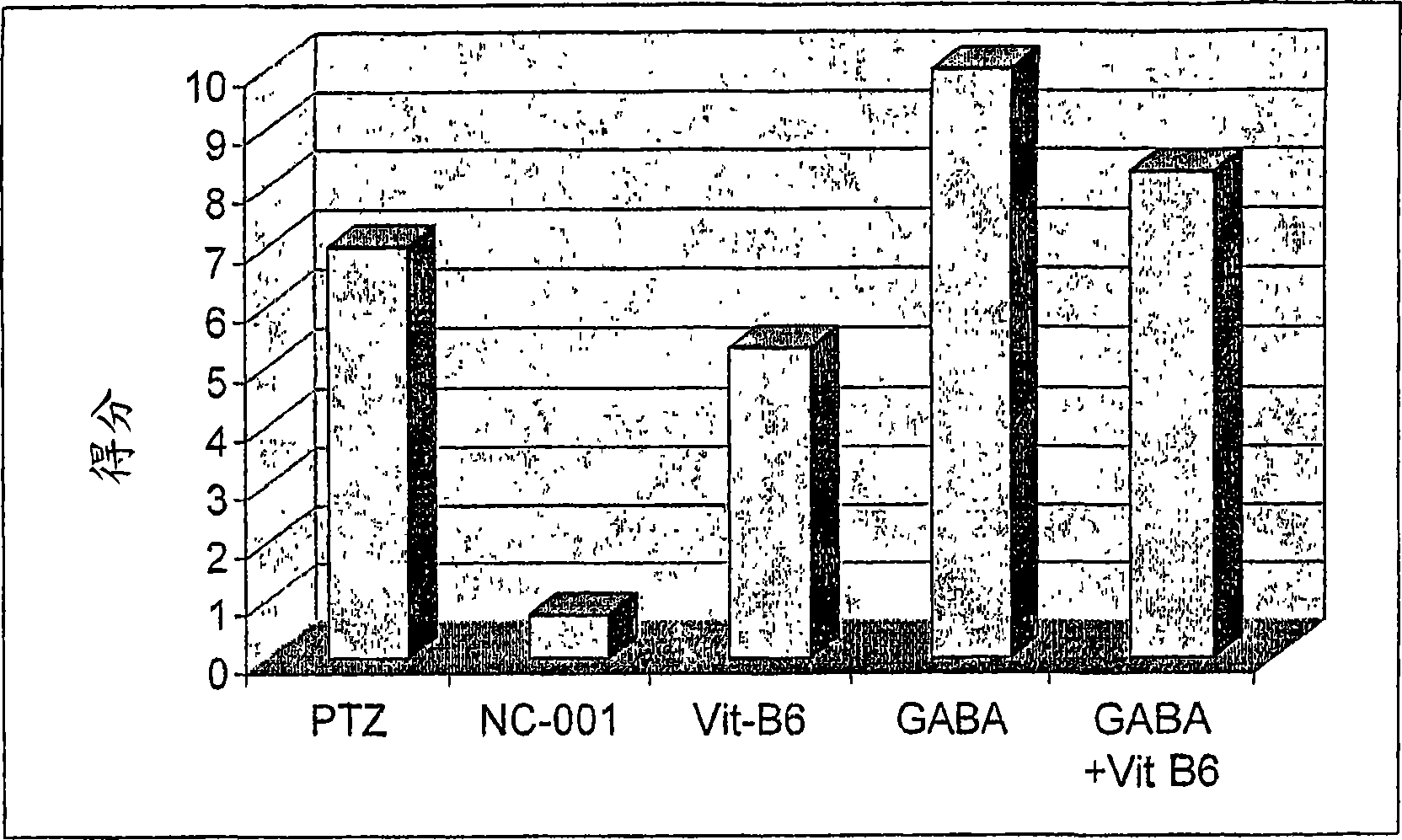
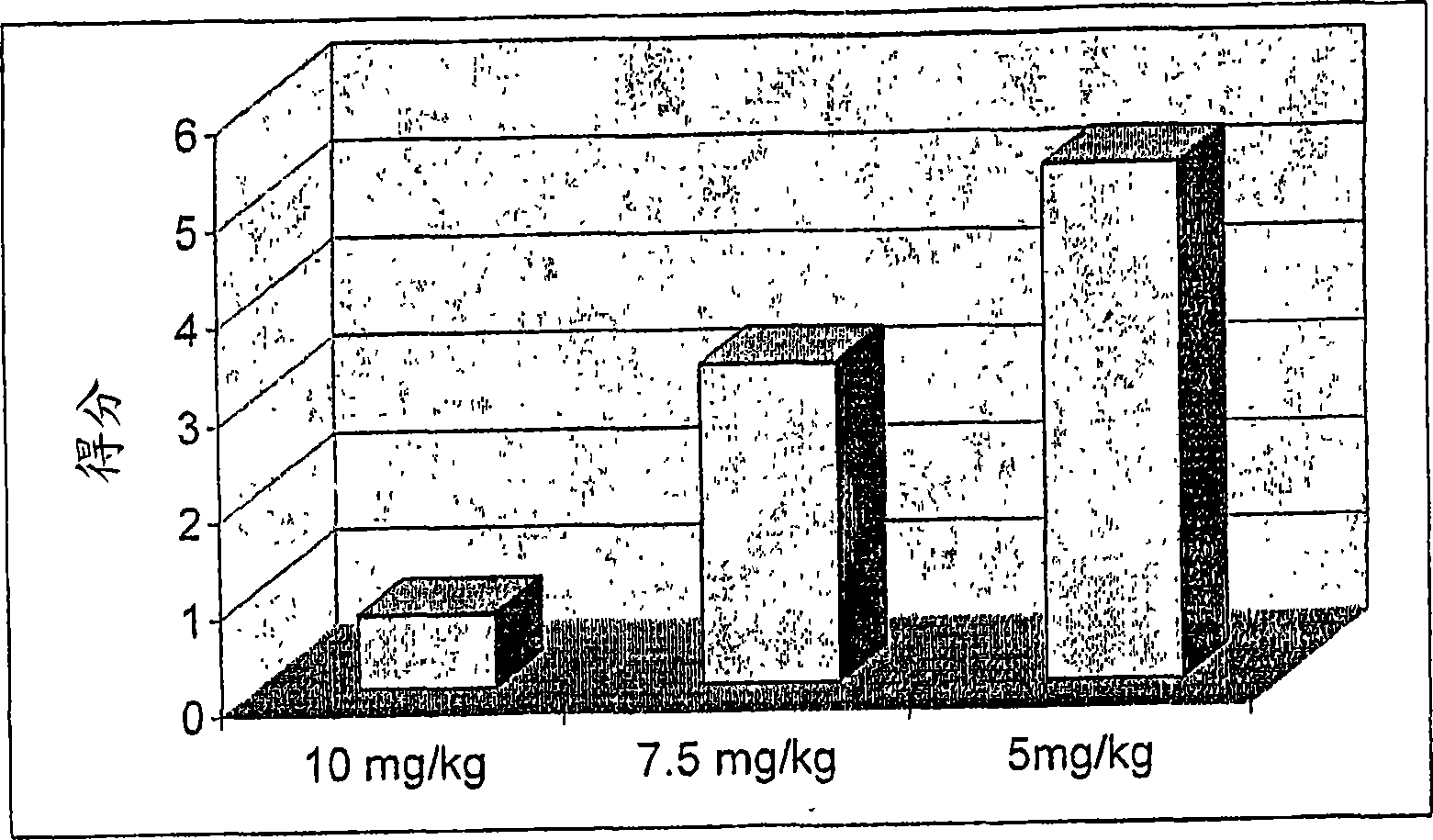
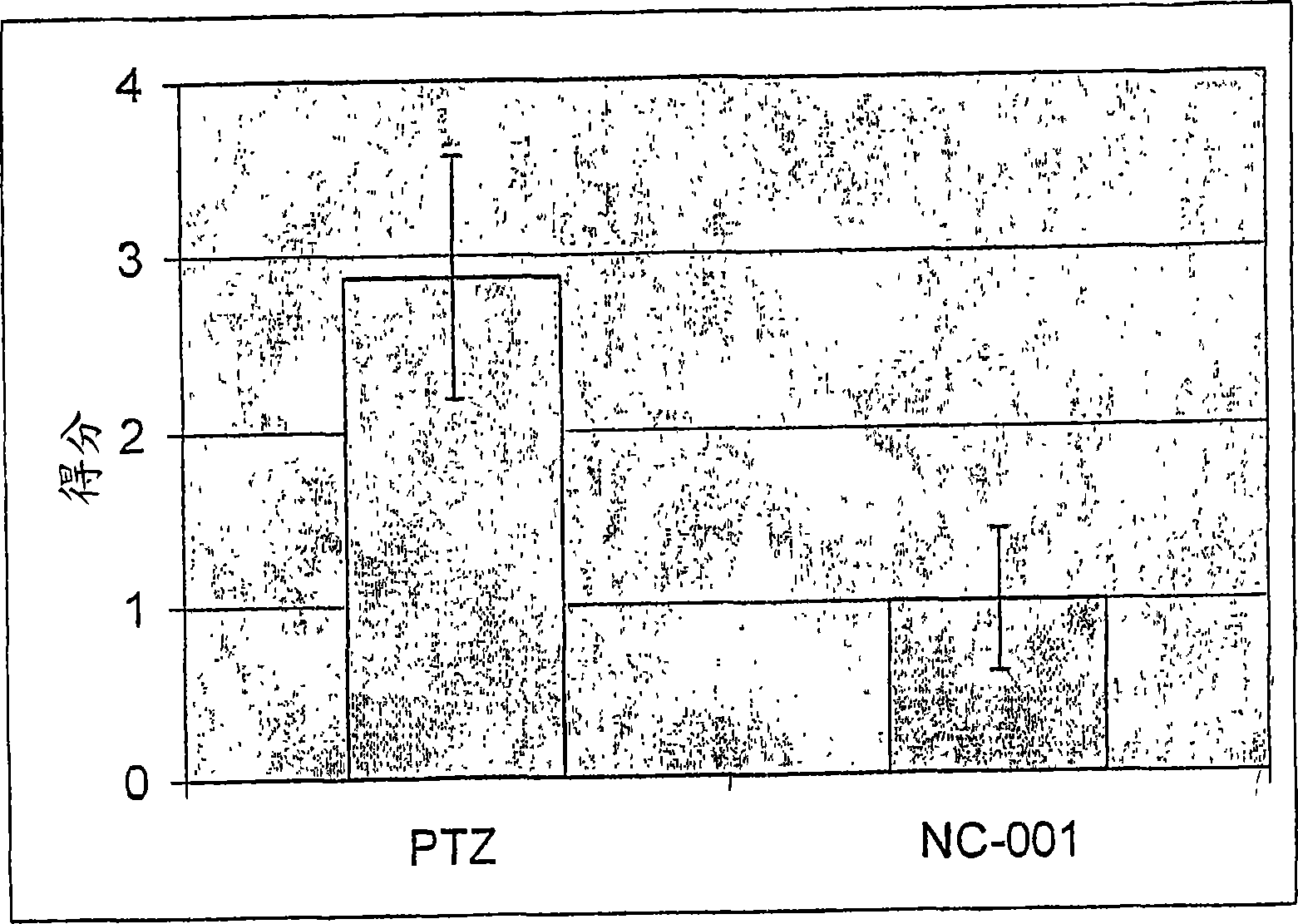
Compositions and methods for reducing the risk of epileptic occurrence and/or for treatment of seizure disorders
A composition and technology of antiepileptic drugs, which can be applied in drug combinations, nervous system diseases, active ingredients of heterocyclic compounds, etc., and can solve problems such as discomfort treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0106] Example 1: Synthesis of [5-hydroxy-6-methyl-4-(hydroxymethyl)-pyridin-3-yl]methyl-4-aminobutyrate, dihydrochloride (=B6-GABA)
[0107] The chemical structure of [5-hydroxy-6-methyl-4-(hydroxymethyl)-pyridin-3-yl]methyl-4-aminobutyrate, dihydrochloride is:
[0108]
[0109] The synthesis of [5-hydroxy-6-methyl-4-(hydroxymethyl)-pyridin-3-yl]methyl-4-aminobutyrate, dihydrochloride was a five-step process. All synthesized compounds were characterized by NMR and mass spectrometry.
[0110] 1.3, the synthesis of 4-bis(bromomethyl)-5-hydroxyl-6-methylpyridine (2):
[0111]
[0112] In the first step, pyridoxine (1) (20 g, 0.097 mol) was refluxed in 48% hydrobromic acid (150 ml) for 1 hour. After crystallization at -15°C the precipitate was isolated, washed with acetone and dried. Yield 25 g (68%).
[0113] MS(ES): m / z295.95; 297.93(M+H) + .
[0114] 2. Synthesis of 3-bromomethyl-5-hydroxy-4-hydroxymethyl-6-methylpyridine hydrobromide (3).
[0115]
[0116] 4,5...
Embodiment 2
[0131] Example 2: [3-(5-hydroxyl-6-methyl-4-(hydroxymethyl)pyridyl]methyl-2-[(4-hydroxyl)quinoline formate (=B6-Kyn) synthesis
[0132] The chemical structure of [3-(5-hydroxy-6-methyl-4-(hydroxymethyl)pyridyl]methyl-2-[(4-hydroxy)quinolinecarboxylate is:
[0133]
[0134] Under argon, a mixture of 4-hydroxyquinoline-2-carboxylic acid hydrate (kynurenic acid (7)) (1.80 g, 8.70 mmol) and cesium carbonate (4.25 g, 13.05 mmol) was stirred at room temperature in Stir in dry DMSO (70ml) for 1.5 hours. 3-Bromomethyl-5-hydroxy-4-hydroxymethyl-6-picoline hydrobromide (3) (3.58 g, 11.42 mmol) was then added. The resulting brown solution was kept at room temperature for 18 hours, then diluted with water (200ml) and extracted with ethyl acetate (12x150ml). After crystallization at 5°C the precipitate was isolated, washed with ethyl acetate, ether and dried. Yield 0.25 g (7.8%).
[0135] 1 H NMR (DMSO-d 6 )δ: 2.36(s, 3H), 4.77(s, 2H), 5.47(s, 2H), 6.60(s, 1H), 7.37(t, 1H), 7.69(...
Embodiment 3
[0137] Example 3: [4-(4-hydroxyquinoline-2-carbonamido)]butanoic acid 5-hydroxyl-4-hydroxymethyl-6-methyl-pyridin-3-yl)methyl ester (B6- GABA-Kyn) synthesis
[0138] The chemical structure of [4-(4-hydroxyquinoline-2-carbonylamino)]butanoic acid 5-hydroxy-4-hydroxymethyl-6-methyl-pyridin-3-yl)methyl ester is:
[0139]
[0140] 1. Synthesis of 4-(4-hydroxyquinoline-2-carbonylamino)butanoic acid (10).
[0141]At 50°C, BSA (N, O-bis(trimethylsilyl)acetamide) (3.26ml, 13.20mmol) was added to 4-aminobutyric acid (GABA) (0.62g, 6.00mmol) in a dry dichloromethane (10ml) and the mixture was stirred for 6 hours. The resulting solution was added in the mixed anhydride which was prepared from kynurenic acid (7) (0.95g, 5.00mmol), Et 3 N (1 ml), EtOCOCl (0.5 ml, 5.25 mmol) was prepared in dry DMF (10 ml) at -20°C for 20 minutes. The reaction mixture was stirred at -5°C for 2 hours and kept at 4°C for a further 18 hours. Water (50ml) and ethyl acetate (30ml) were then added. A mix...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com