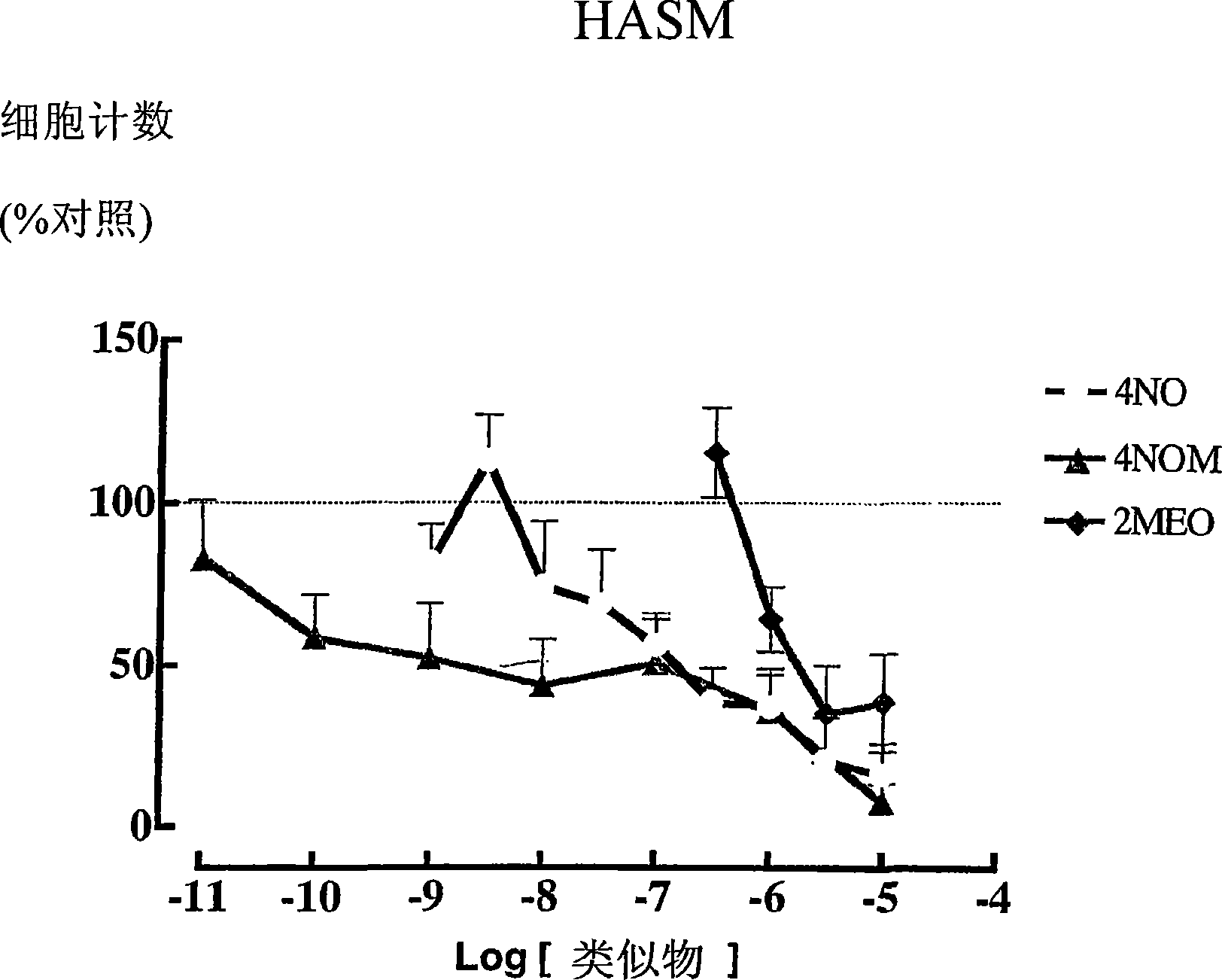
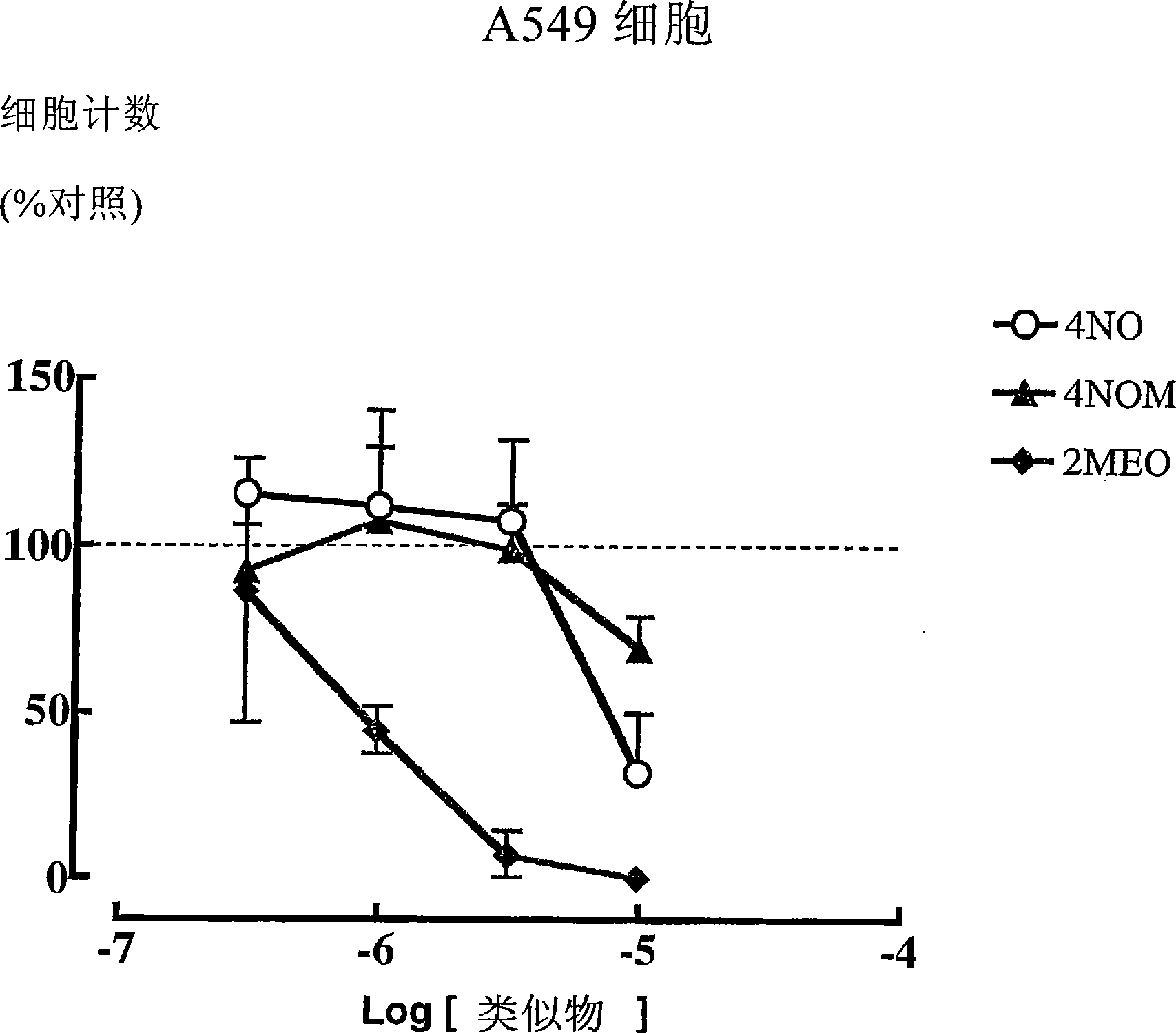
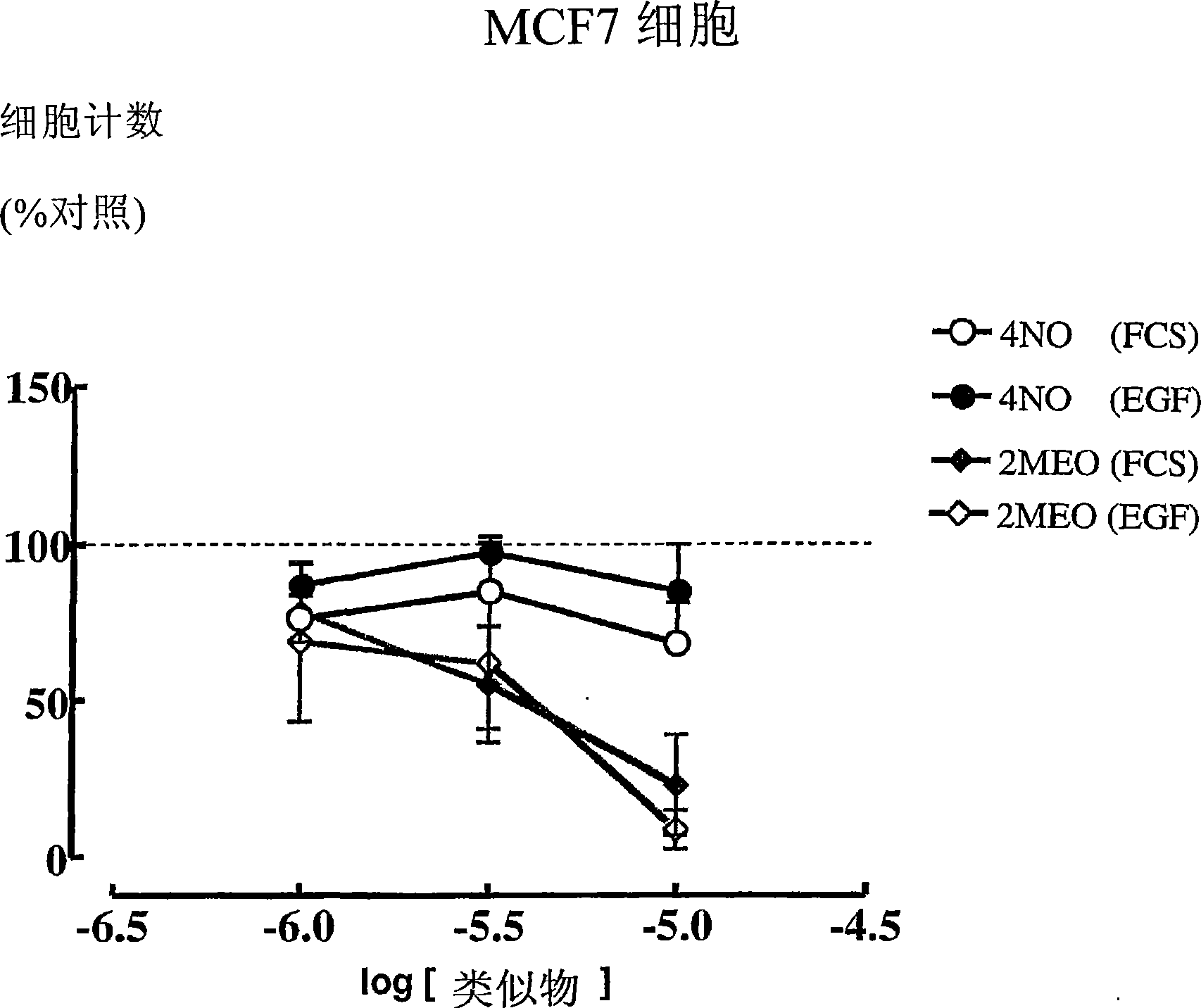
Estratriene derivatives
A technology of derivatives and compounds, applied in the field of estriene derivatives, can solve problems such as airway narrowing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0266] Synthesis of 2-methoxy-6-(4'-nitrobenzyloxy)iminoestradiol ("4NO")
Embodiment 1a
[0268] 2-Methoxyestradiol (1) was diacetylated by treatment with acetic anhydride in pyridine in 95% yield. Benzyl oxidation of this double protected species was performed with chromium trioxide in an acetic acid / water mixture in 45% yield. Both acetates were removed in 98% yield by treating the newly formed ketone compound (3) with potassium carbonate in aqueous methanol. Condensation of the ketone with O-(4-nitrobenzyloxy)hydroxylamine in methanol is then carried out to give 2-methoxy-6-(4-nitrobenzyloxy)iminoestradiol (5), Yield 99%.
[0269]
[0270] Route 9
Embodiment 1b
[0272] 3,17-bis-acetoxy-2-methoxyestra-1,3,5(10)triene (2)
[0273] 2-Methoxyestradiol (127.5 mg, 0.426 mmol) was dissolved in anhydrous pyridine (6.0 mL) under nitrogen atmosphere and cooled to 0°C. Acetic anhydride (3.0 mL) was added dropwise, and the reaction was allowed to warm to room temperature. After stirring overnight, the reaction mixture was cooled to 0 °C, at which point 50.0 mL of 1M aqueous hydrochloric acid was added and the reaction was allowed to warm to room temperature. Extraction was performed with ethyl acetate (3 x 50 mL), and the combined organic extracts were washed successively with 3M aqueous hydrochloric acid (50 mL), water (50 mL) and brine (50 mL). The organic layer was dried over sodium sulfate, then filtered, and the solvent was concentrated in vacuo to give a white solid (154 mg). The product was homogeneous when analyzed by TLC, so purification was deemed unnecessary, but an analytical sample was purified on a silica gel column using 1:5 ethy...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com