Gambogicacid derivative and its preparation method and uses in pharmacy
A Derivative, Gambogic Acid Technology, Applied in Pharmaceutical Field
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
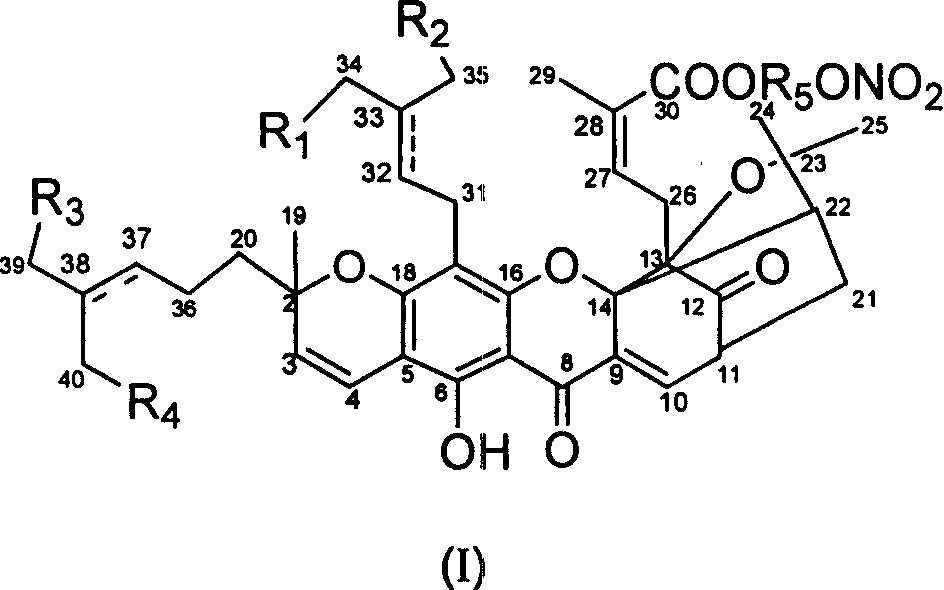
[0064] Embodiment 1: Gambogic acid-2'-nitrooxyethyl ester (I-1)
[0065] Dissolve 250mg (0.4mmol) of gambogic acid in 15ml of acetone, add 0.18ml (2.08mmol) of dibromoethane and 0.4ml of triethylamine respectively at room temperature, the reaction solution is yellow and transparent, after the addition, reflux for 6 hours , concentrated the reaction solution directly, put it on the column by dry method, column chromatography, eluting with petroleum ether / ethyl acetate (4:1), and prepared 80 mg of yellow oil (A-1) with a yield of about 27%. Then, 80mg (0.11mmol) of compound (A-1) was dissolved in 10ml (1:1) of anhydrous tetrahydrofuran and acetonitrile mixed solvent, and 28mg (0.17mmol) of silver nitrate was added at room temperature, and the reaction was done under reflux in the dark for 5 hours. , the reaction solution was filtered to remove the precipitate, the filtrate was concentrated, put on the column by dry method, column chromatography, petroleum ether / ethyl acetate (4:...
Embodiment 2
[0069] Embodiment 2: Gambogic acid-3'-nitrooxypropyl ester (I-2)
[0070] With reference to the preparation method of compound (A-1), the yellow oil (A-2 ) 750mg, yield 95%. Referring to the preparation method of compound I-1, 710 mg (0.95 mmol) of compound (A-2) and 242 mg (1.43 mmol) of silver nitrate were refluxed and reacted, and the aftertreatment was the same as above to obtain 140 mg of yellow sticky substance (I-2). Yield 20%.
[0071] IR(KBr): v=3456, 2967, 2924, 2858, 1736, 1710, 1632, 1593, 1438, 1383, 1331, 1279, 1228, 1177, 1140, 1048cm -1 .
[0072] 1 HNMR (300M, CDCl 3 ): δ12.86(1H, s, 6-OH), 7.56(1H, brs, H-10), 6.67(1H, m, H-4), 6.14(1H, m, H-27), 5.45( 1H, m, H-3), 5.06 (2H, m, H-32, H-37), 4.39 ((2H, m, H-1'), 3.90 (2H, m, H-3'), 3.48 (3H, m, H-2', H-11), 3.30 (1H, m, H-31a), 3.23 (1H, m, H-31b), 2.98 (2H, dd, J=9.7Hz, 7.9Hz , H-26), 2.52 (1H, m, H-22), 2.36 (1H, dd, J=13.0Hz, 4.6Hz, H-21a), 2.03 (2H, m, H-36), 1.75 (3H , s, H-25), 1.72 (2H, m, H-20...
Embodiment 3
[0074] Embodiment 3: Gambogic acid-4'-nitrooxybutyl ester (I-3)
[0075] With reference to the preparation method of compound (A-1), yellow oil (A -3) 630mg, yield 83%. Referring to the preparation method of compound (I-1), 630mg (1.22mmol) of compound (A-3) and 311mg (1.83mmol) of silver nitrate were refluxed, and the aftertreatment was the same as above to obtain a yellow sticky substance (I-3) 160 mg, yield 39%.
[0076] IR(KBr): v=3455, 2967, 2924, 2858, 1736, 1710, 1632, 1593, 1438, 1382, 1331, 1278, 1228, 1176, 1140, 1048cm -1 .
[0077] 1 HNMR (300M, CDCl 3 ): δ12.86(1H, s, 6-OH), 7.56(1H, brs, H-10), 6.67(1H, m, H-4), 6.11(1H, m, H-27), 5.45( 1H, m, H-3), 5.07 (2H, m, H-32, H-37), 4.54 ((2H, m, H-1'), 3.53 (4H, m, H-2', H- 3'), 4.10 (2H, m, H-4'), 3.48 (1H, m, H-11), 3.30 (1H, m, H-31a), 3.21 (1H, m, H-31b), 2.98 (2H, dd, J=9.7Hz, 7.9Hz, H-26), 2.51 (1H, m, H-22), 2.36 (1H, dd, J=13.0Hz, 4.6Hz, H-21a), 2.02( 2H, m, H-36), 1.75 (3H, s, H-25), 1.71 (2H, m, H-20)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com