High discharge capacity lithium battery
A battery and capacity technology, applied in the field of electrochemical batteries, can solve problems affecting battery electrical characteristics and discharge characteristics, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
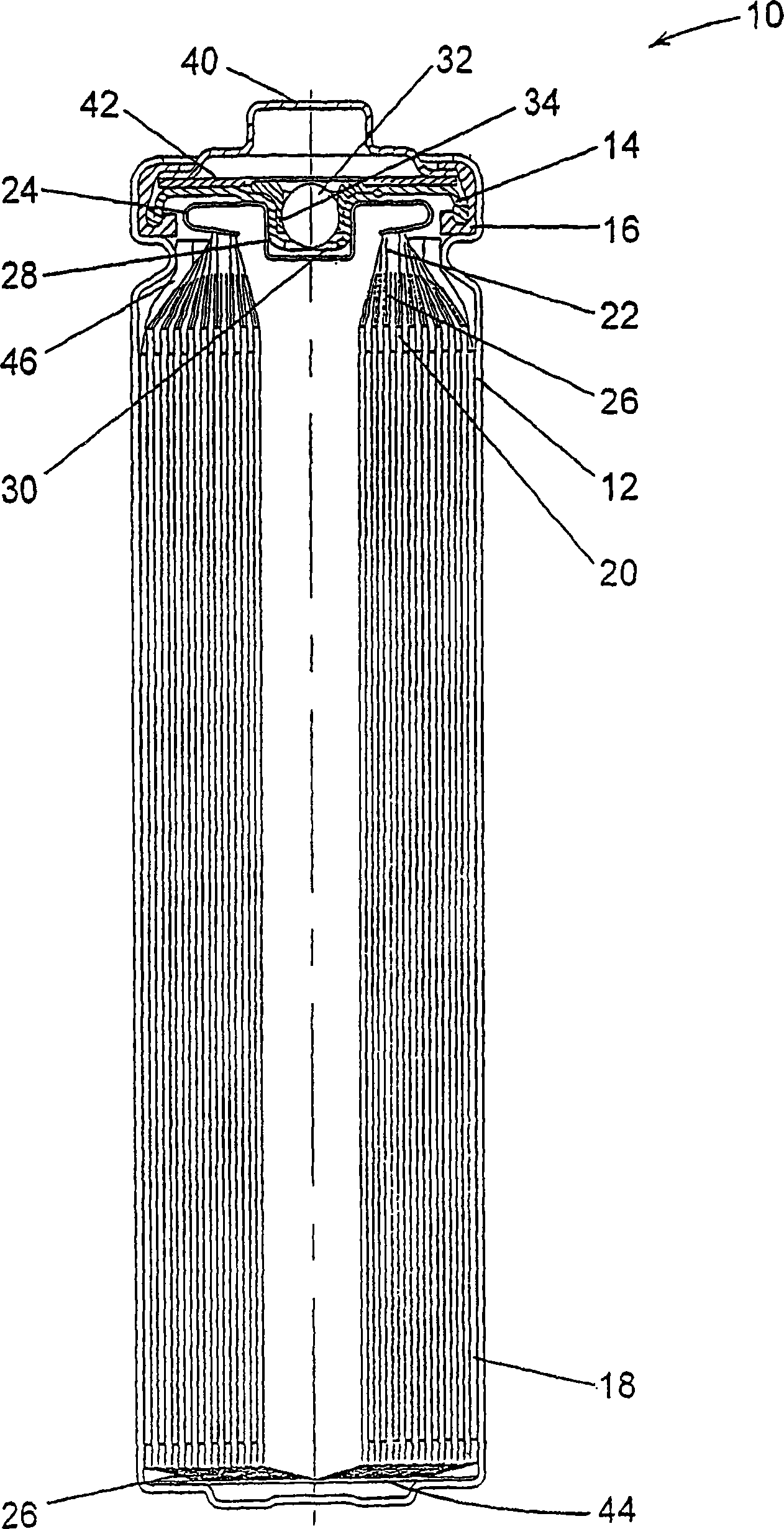
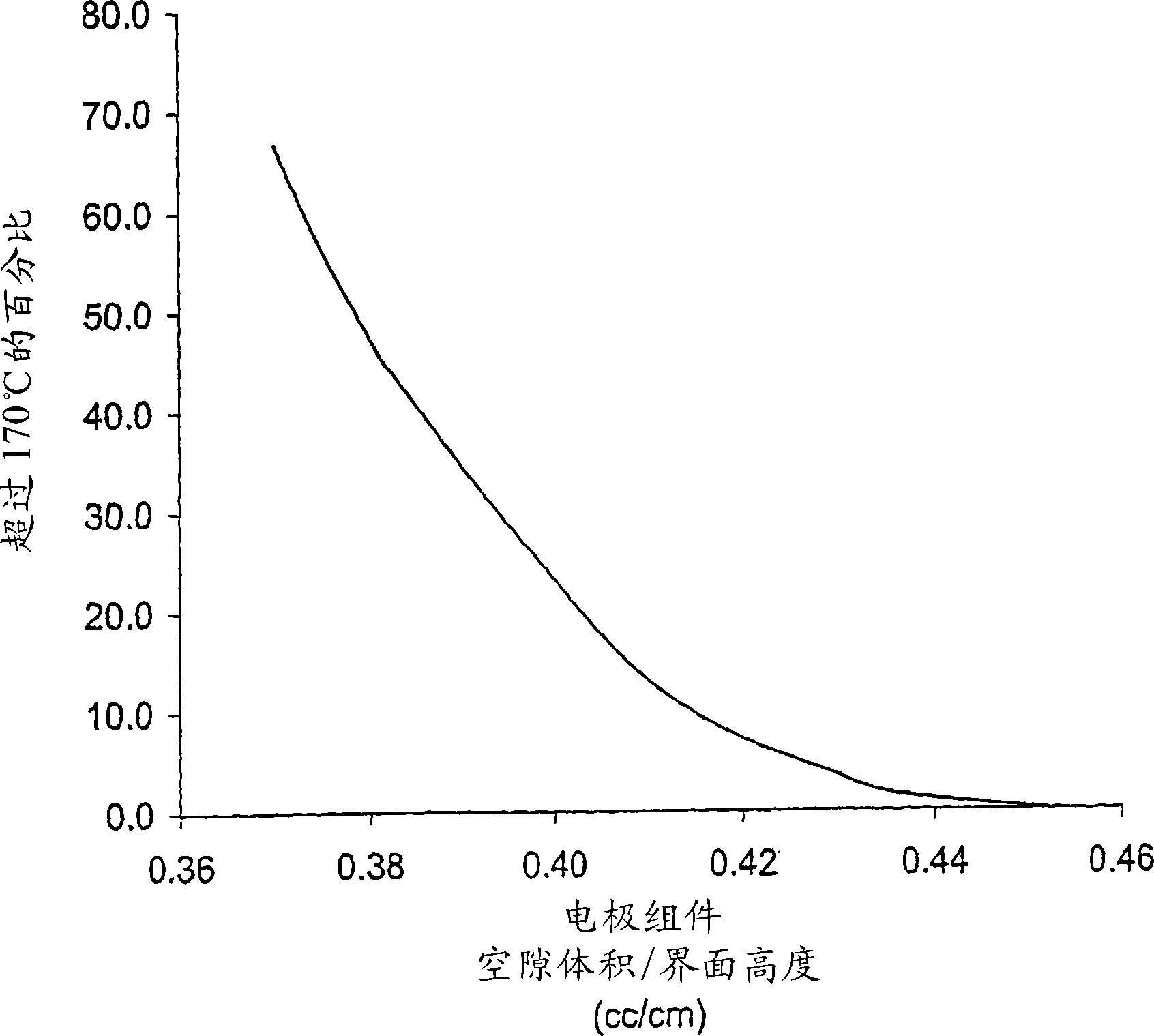
[0076] Fabricate an electrode assembly void volume of about 0.373 to about 0.455 cm per centimeter of interfacial electrode assembly height 3 FR6-type cylindrical Li / FeS with helically wound electrode assemblies varying in the range of / cm 2 Battery. Void volume is varied by adjusting the volume of voids within the active material mixture coated on the cathode. This is done by utilizing various combinations of mixture formulation, thickness and compaction. The separator material used in all cells is a highly crystalline, non-axially oriented microporous polypropylene material with a nominal thickness of 25 μm.
Embodiment 2
[0078] A battery sample of Example 1 was prepared for testing. For each set of cells with a given void volume per unit height, some cells were left undischarged and some cells were discharged 50% (the time required to discharge at a rate of 200mA to remove 50% of the rated capacity). Undischarged and 50% discharged batteries were tested in the impact test, and the external temperature of each of the tested batteries was monitored during the test and for 6 hours after the test.
[0079] For the impact test, place the sample battery on a flat surface, place a 15.8 mm diameter rod along the center of the sample, and drop a 9.1 kg mass onto the sample from a height of 61 ± 2.5 cm. The sample cell was impacted with its longitudinal axis parallel to the flat surface and perpendicular to the longitudinal axis of a 15.8 mm diameter rod placed along the center of the cell. Each sample was hit only once.
[0080] None of the undischarged batteries had an external temperature exceeding...
Embodiment 3
[0084] Four batches of FR6 cells were manufactured, each with a separator made of a different material. A description of the separator materials is provided in Table 1 and typical separator properties are summarized in Table 2, as measured by the method described below. The separator material used in Batch A was the same as that used in the Example 1 cell. Each cell contained about 1.60 g of electrolyte consisting of 9.14 wt% LiI salt in a solvent mixture including 1,3-dioxolane, 1,2-dimethoxyethane and 3, 5-Dimethylisoxazole (63.05:27.63:0.18 by weight).
[0085] Batch A
[0086] Nature (unit)
[0087] The same battery design was used for all batches A-D. The cell was designed with a larger amount of active material, higher FeS 2 Concentration and increased electrode interfacial surface area and lower anode:cathode total input capacity ratio, which resulted in a 22% increase in battery interfacial capacity.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com