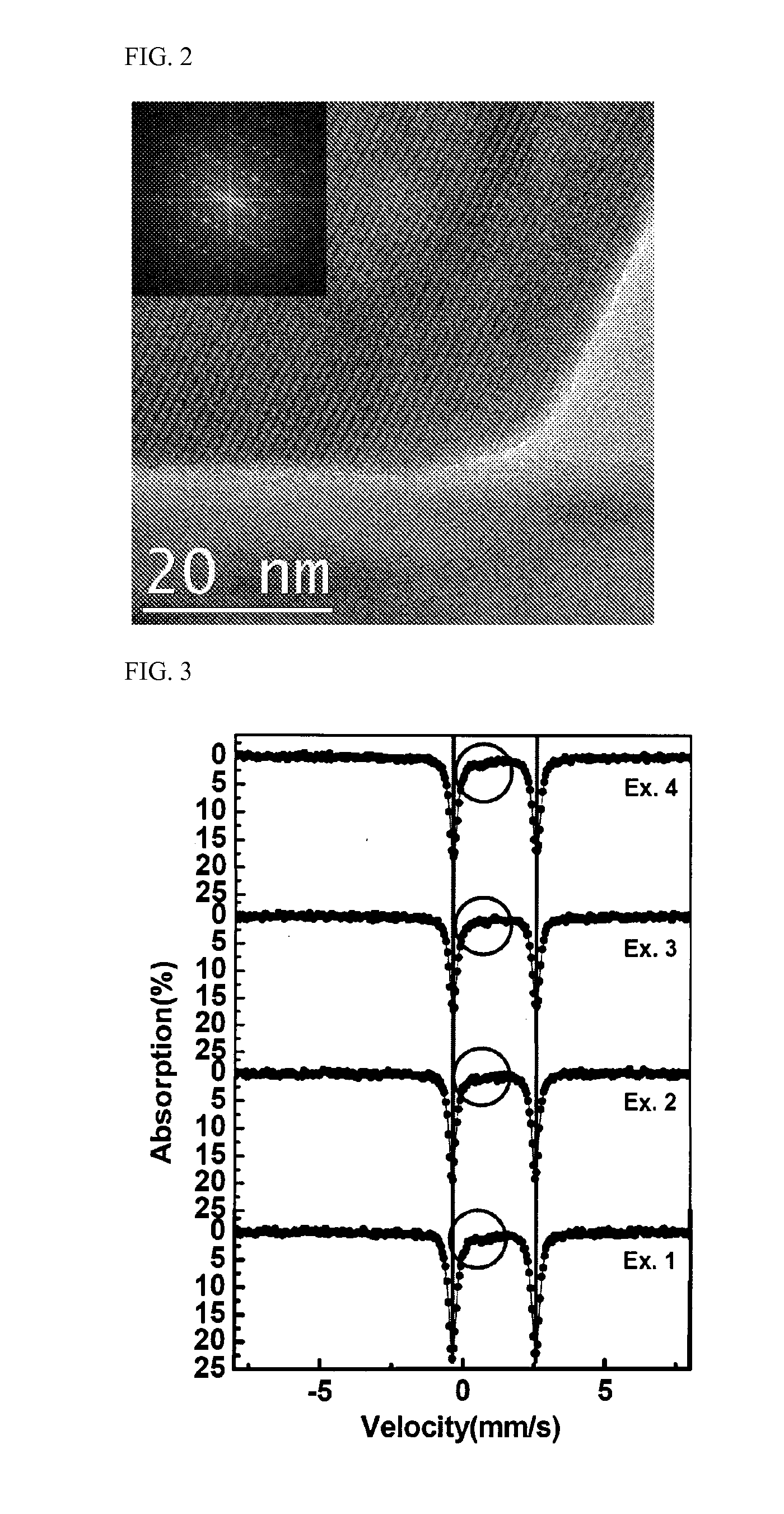
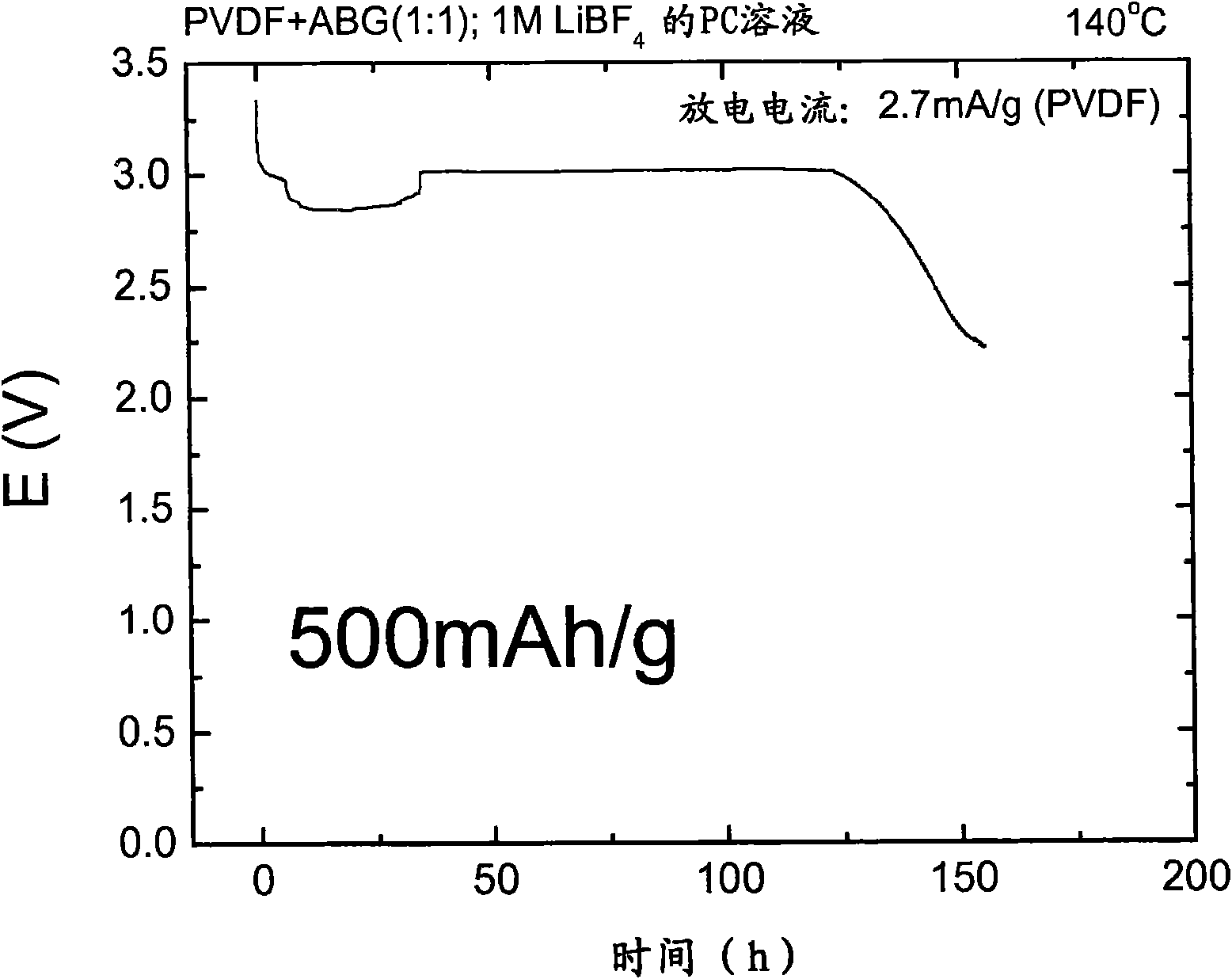
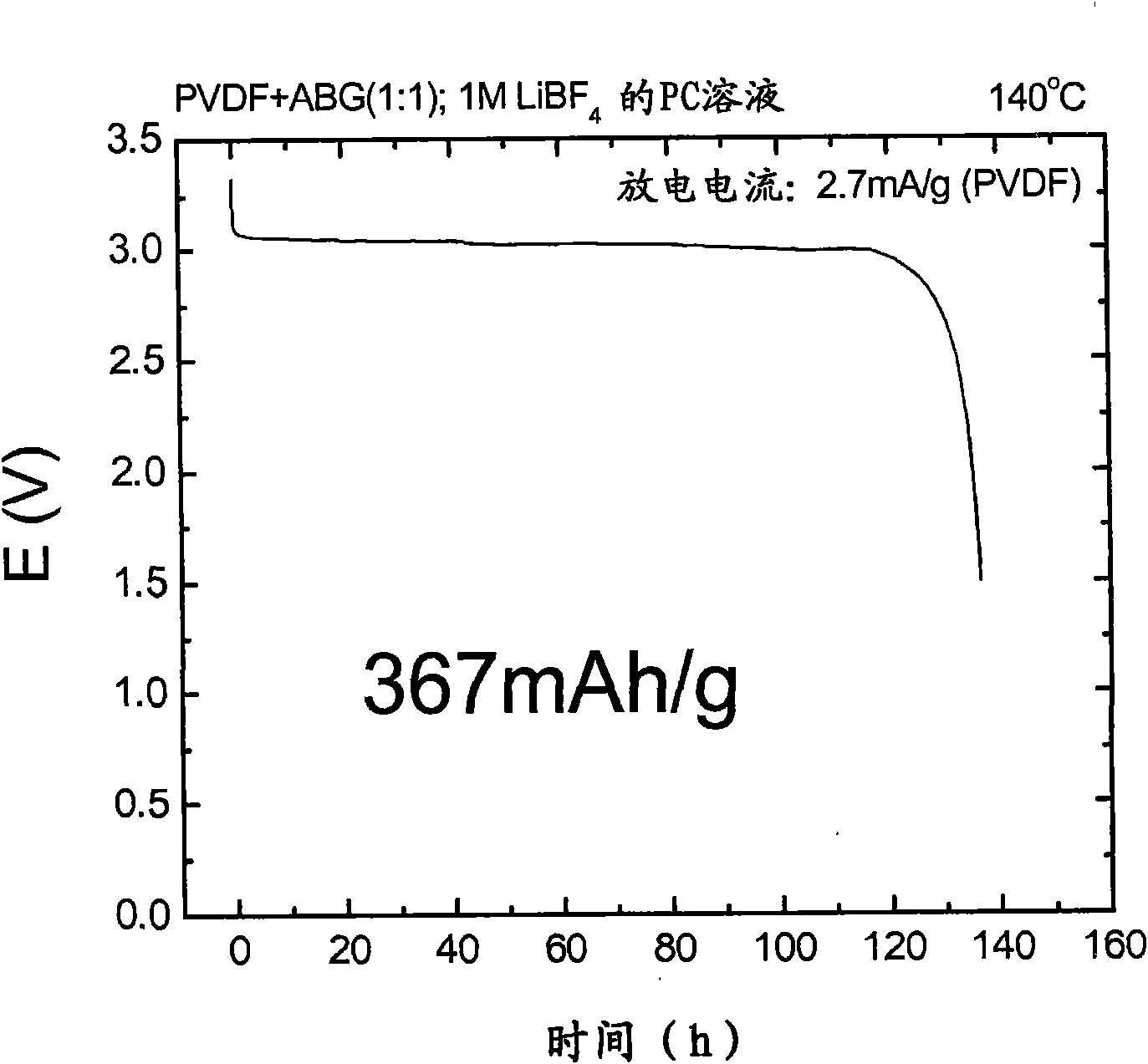
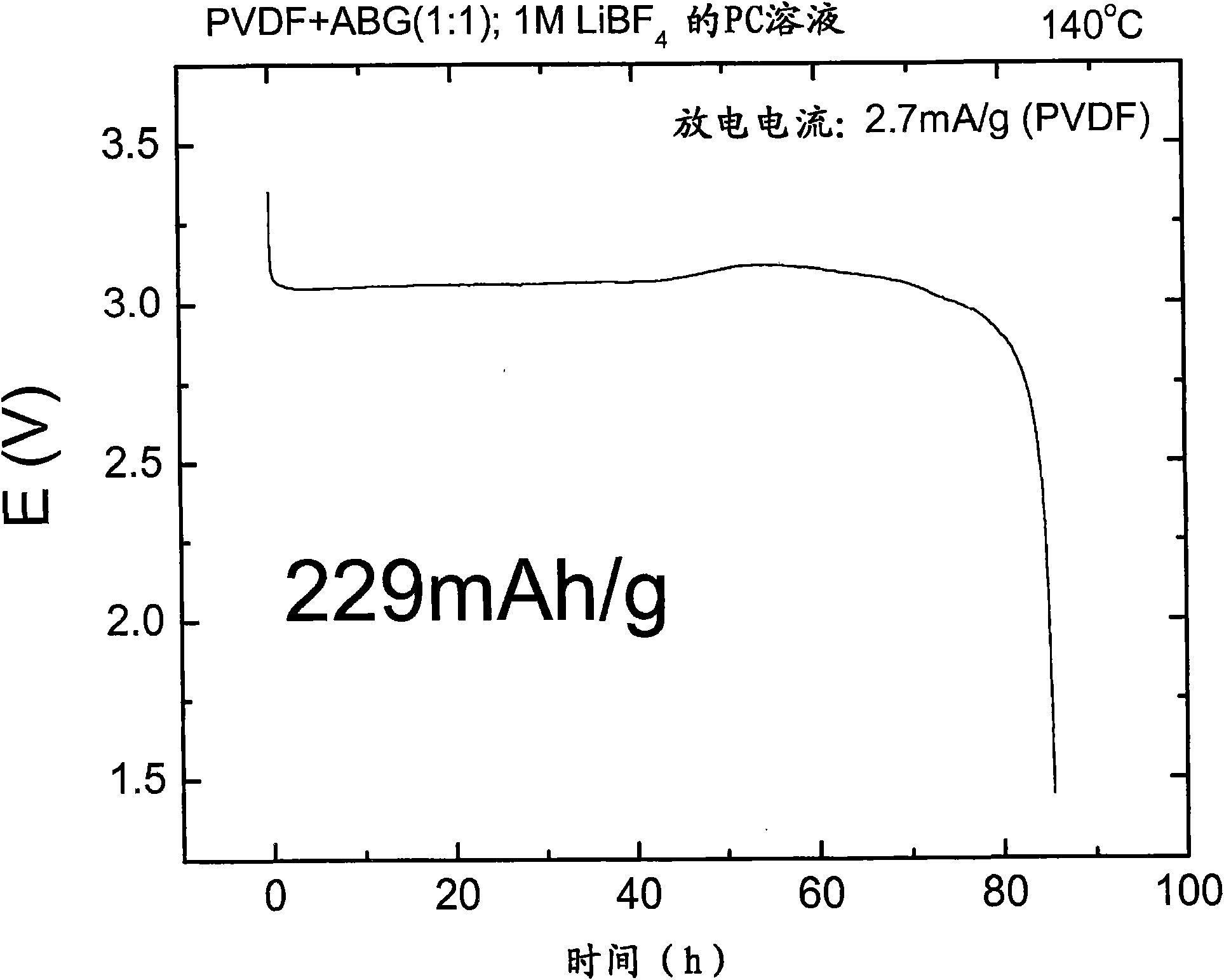
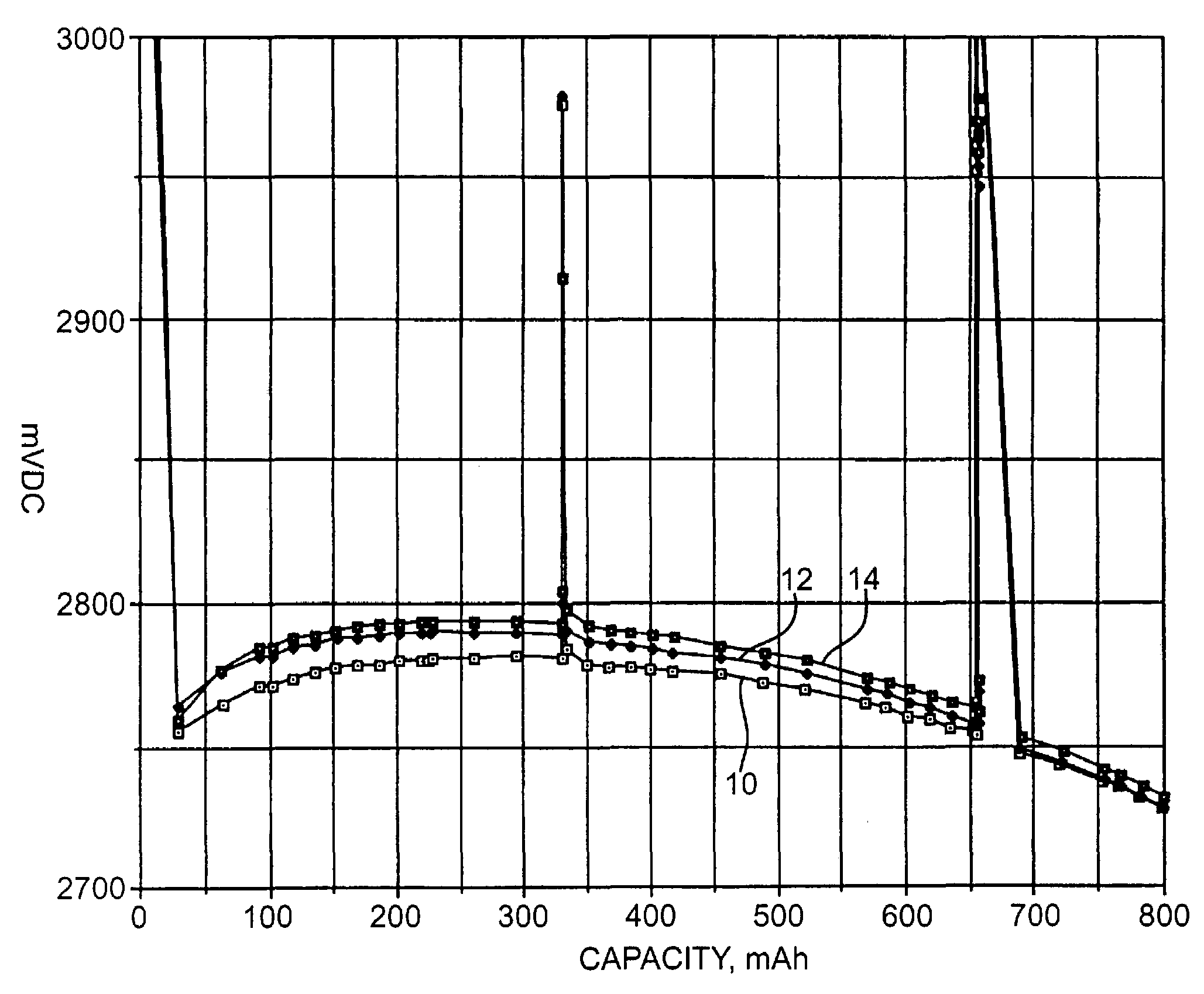
Patents
Literature
50results about "Electrolyte/electrodes capacity ratio" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
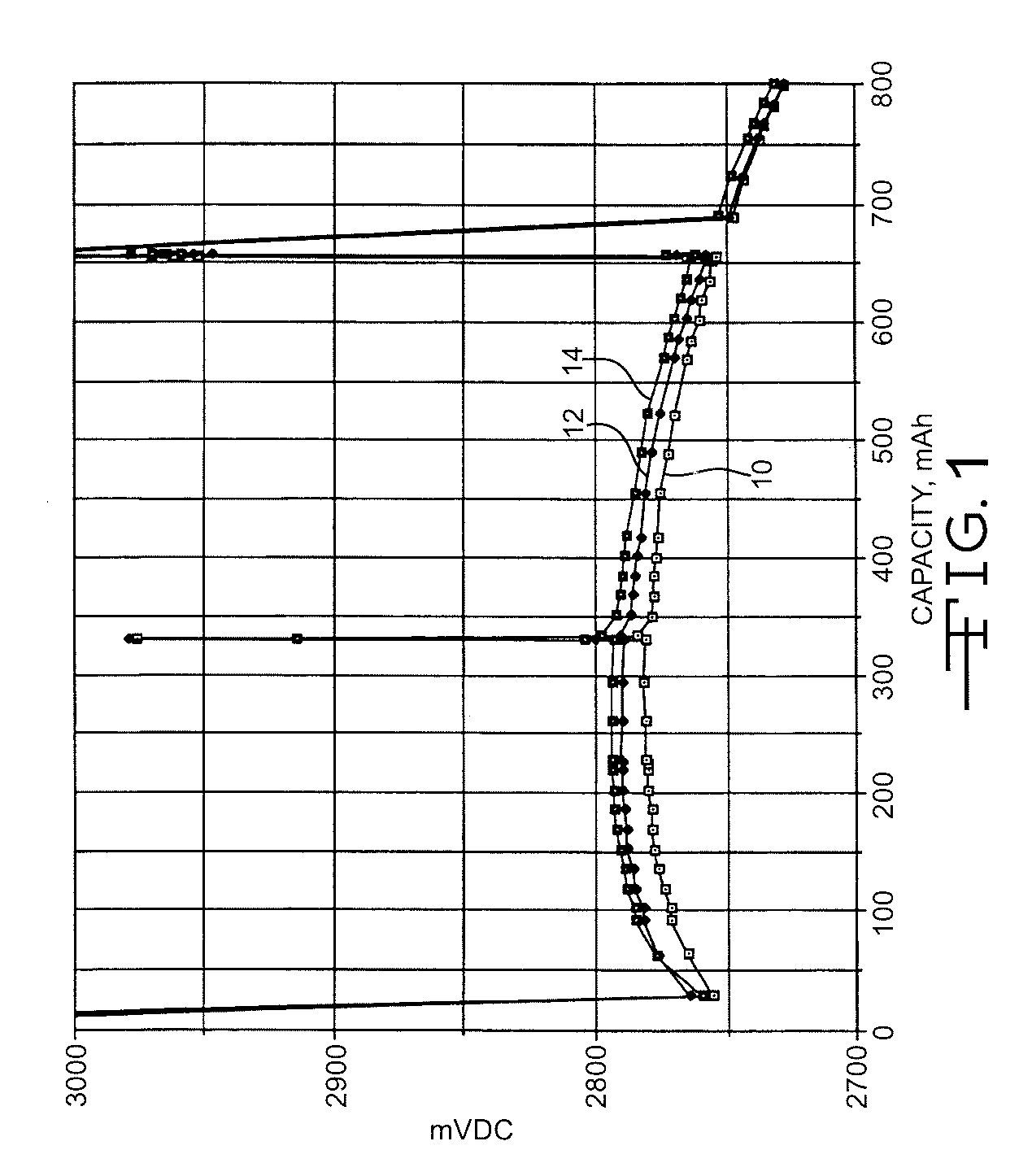
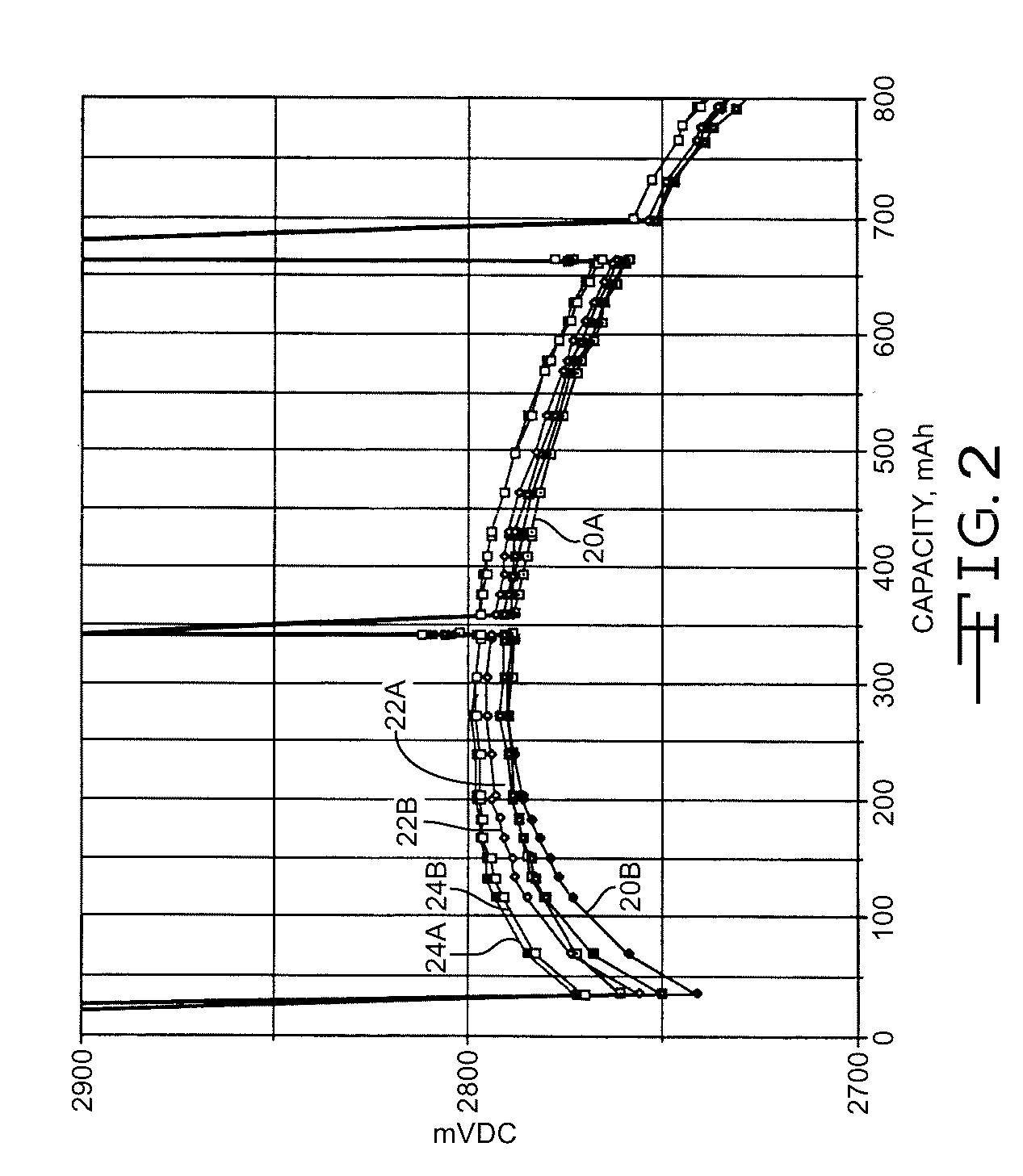
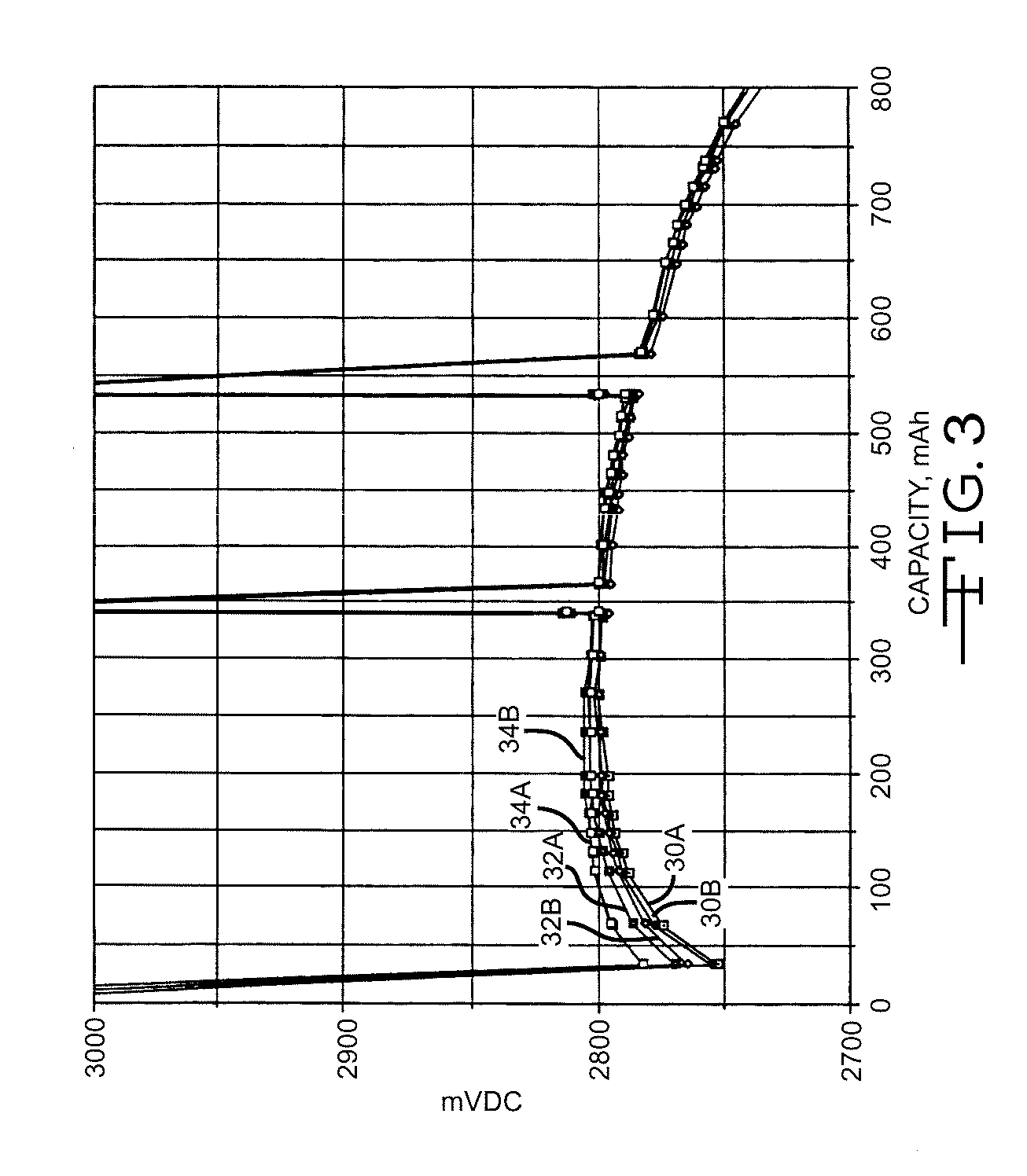
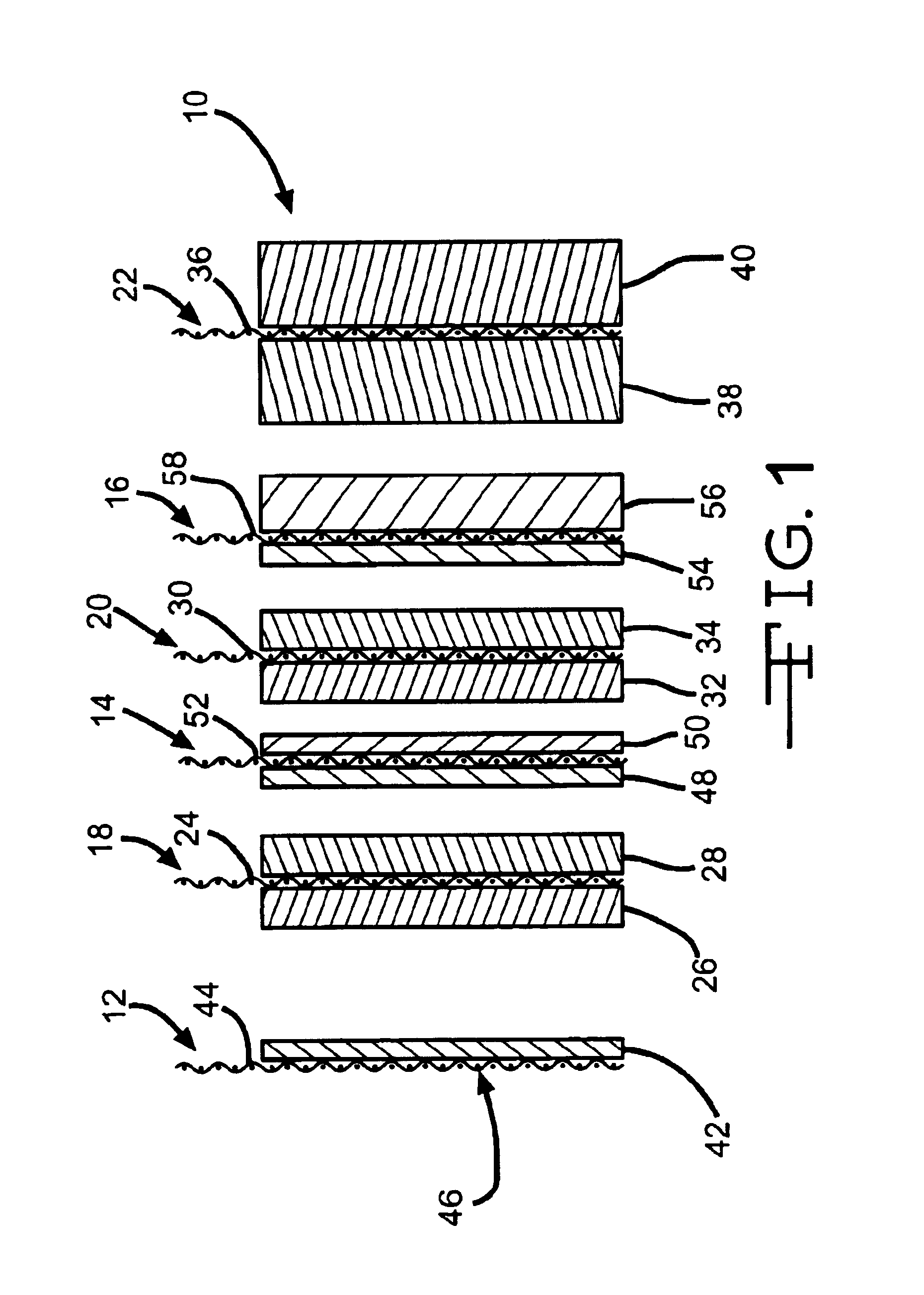
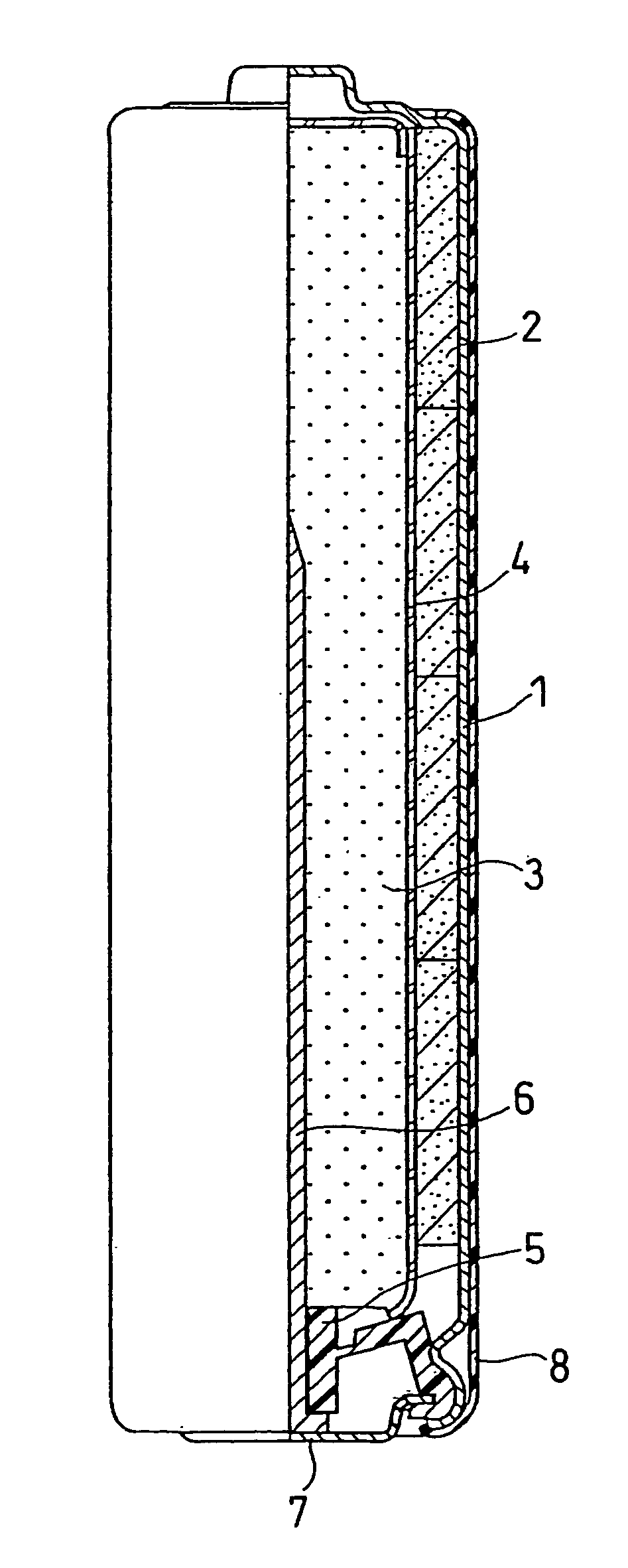
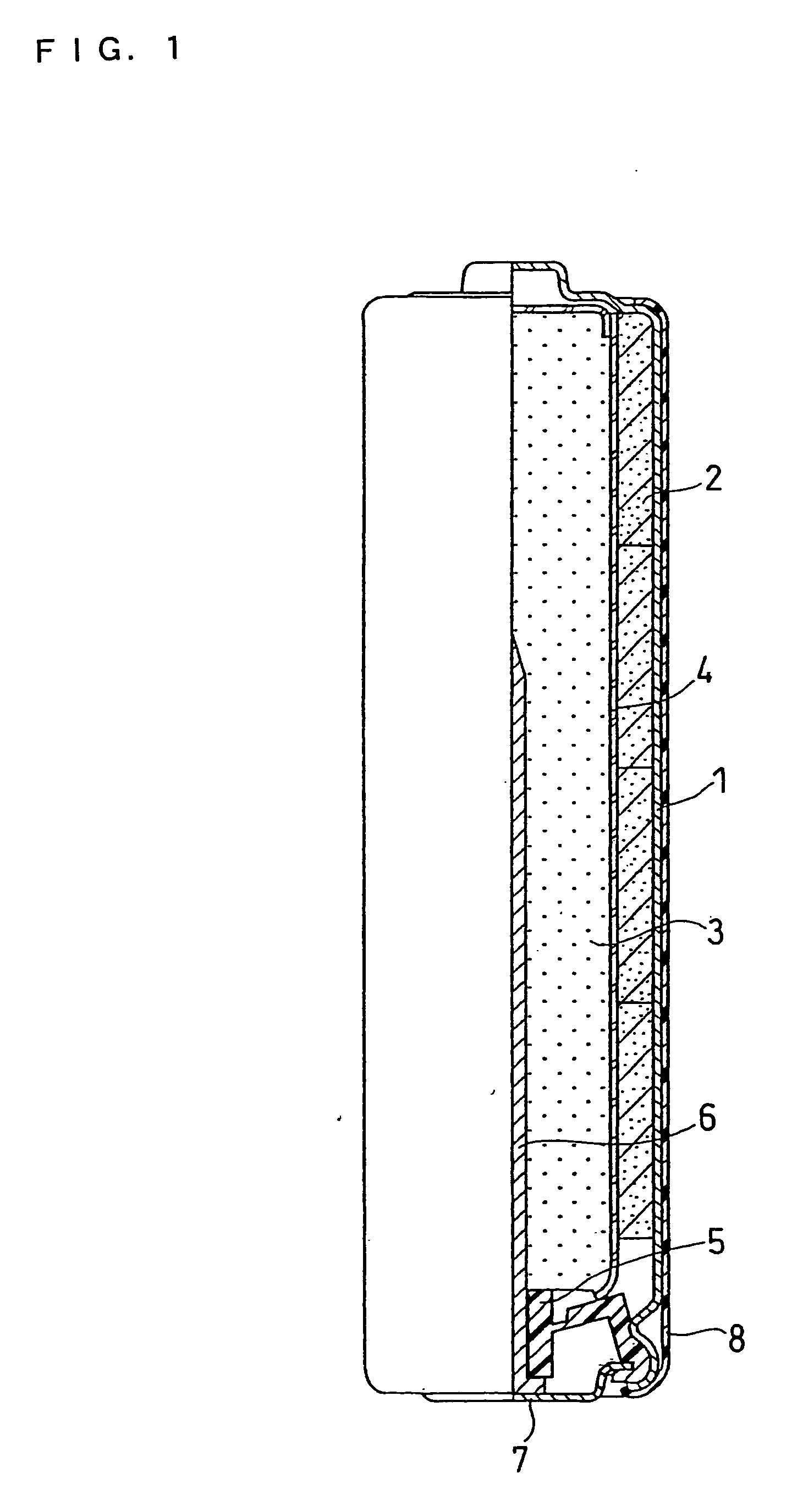
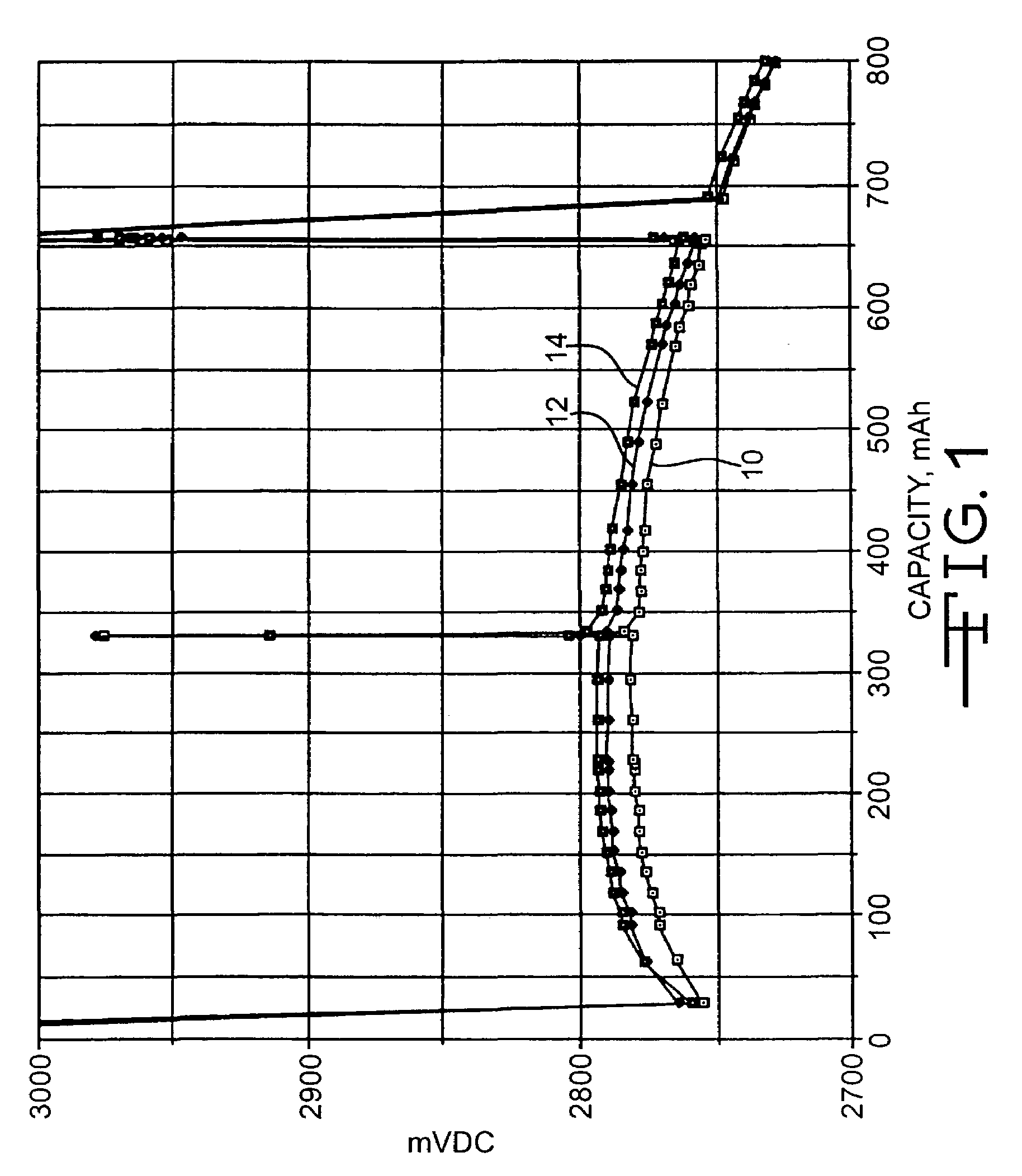
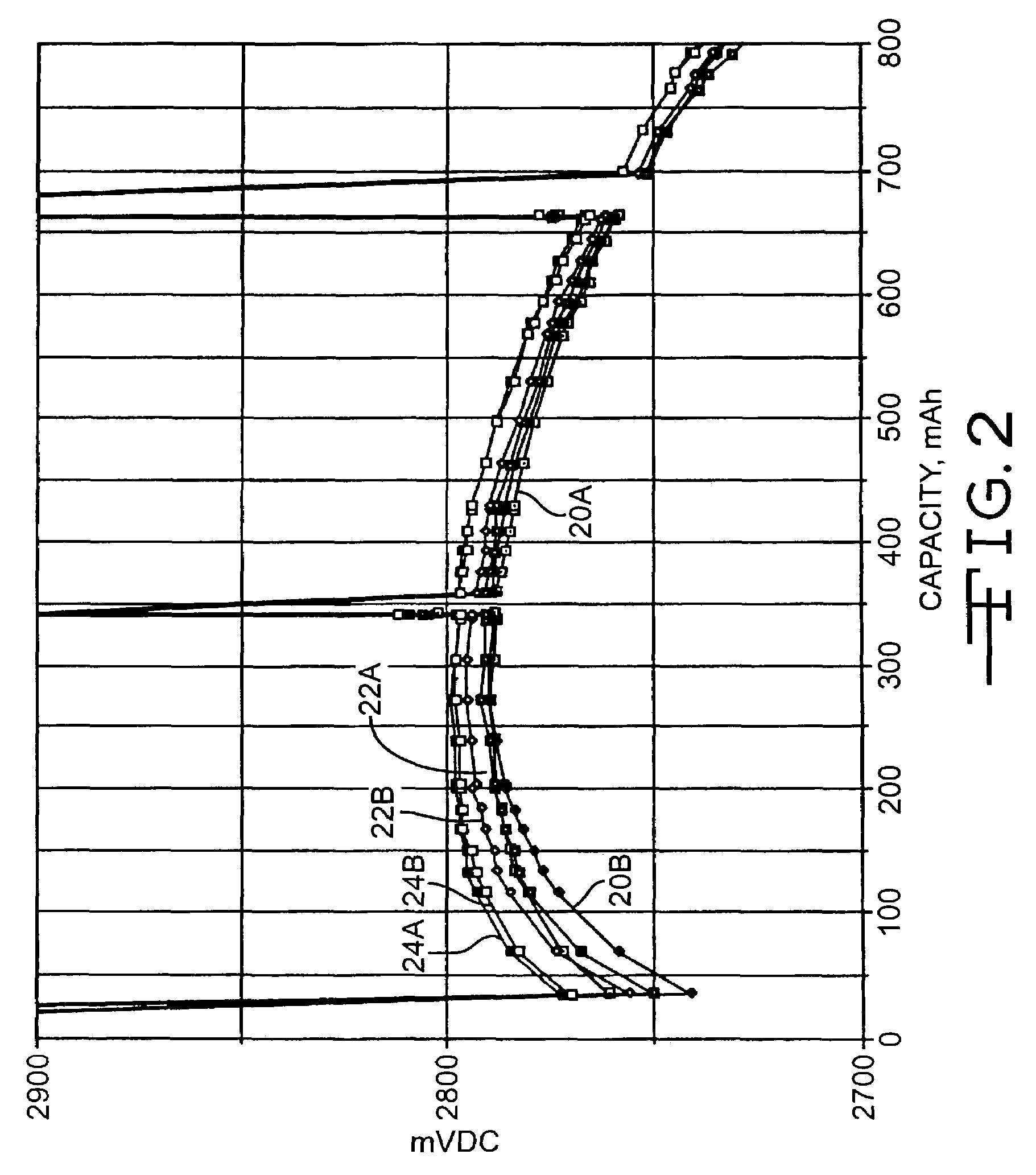
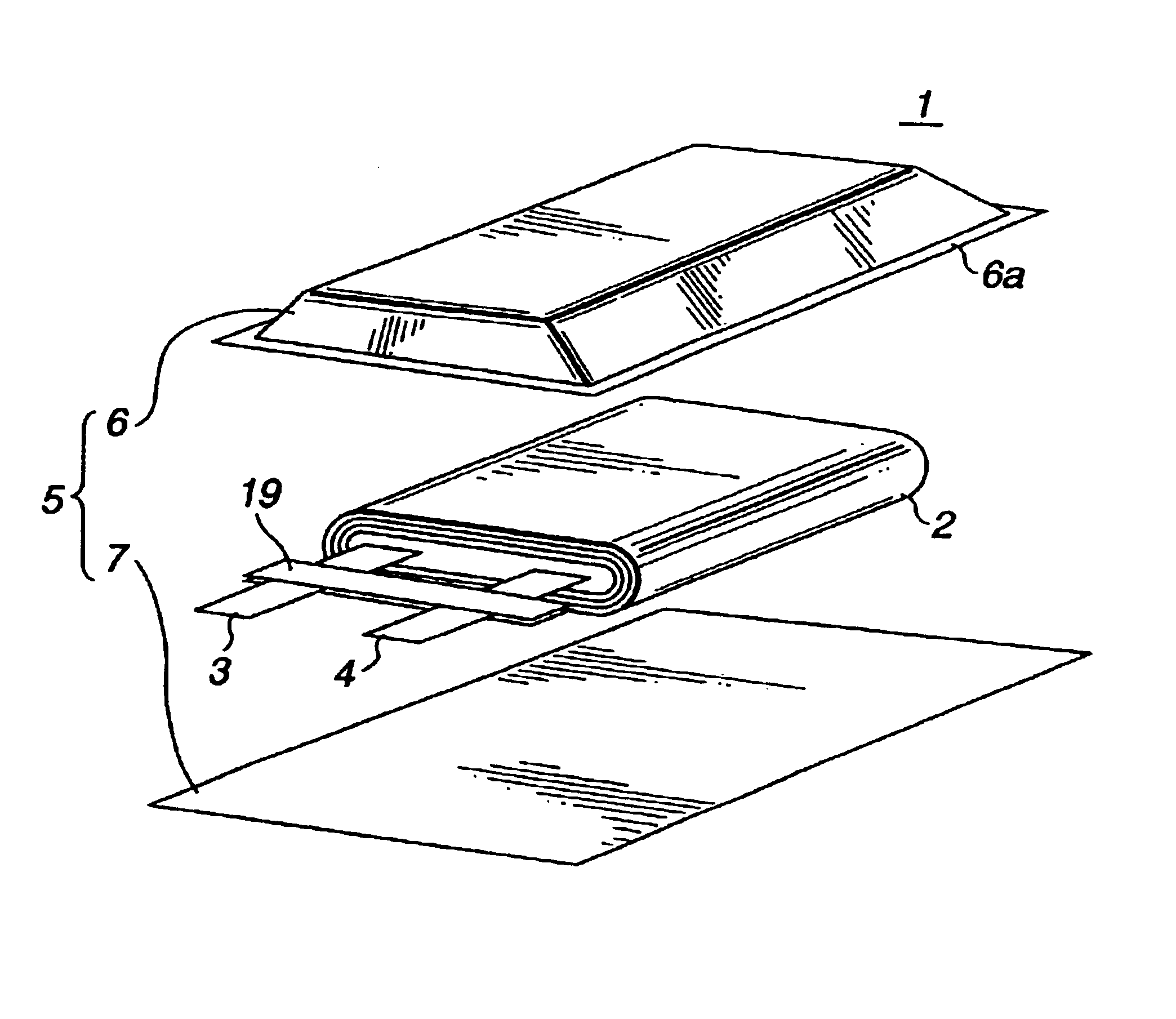
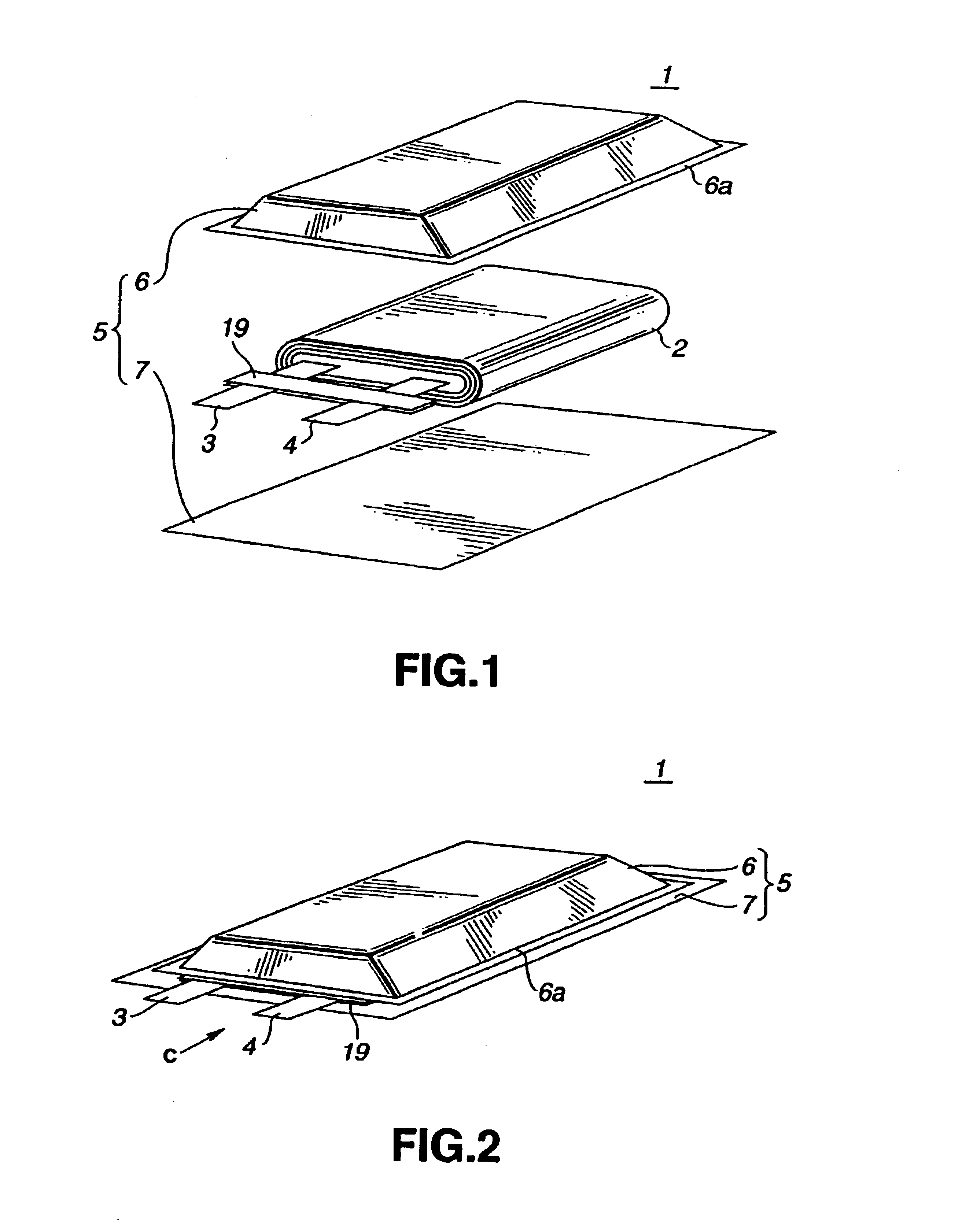
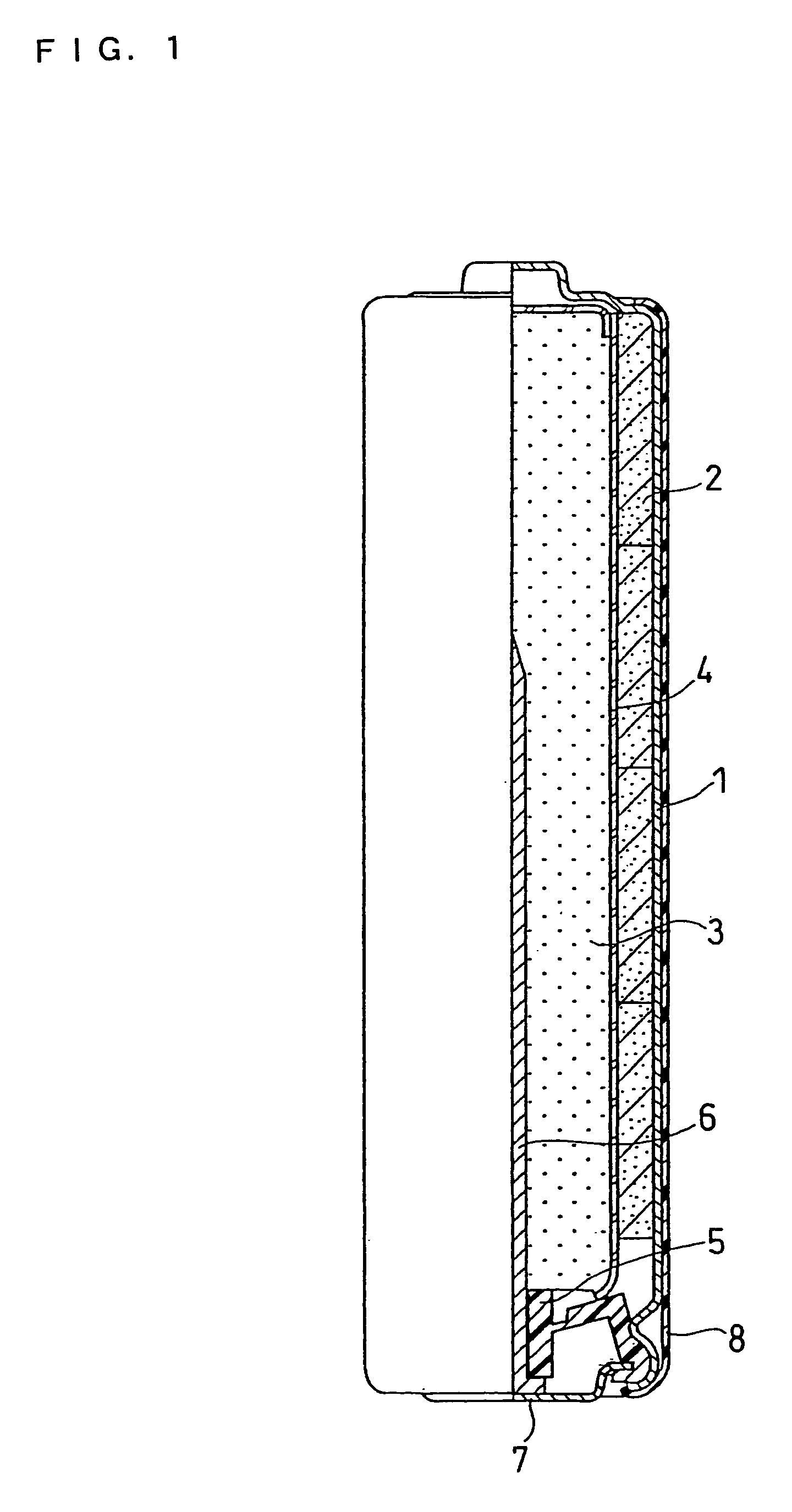
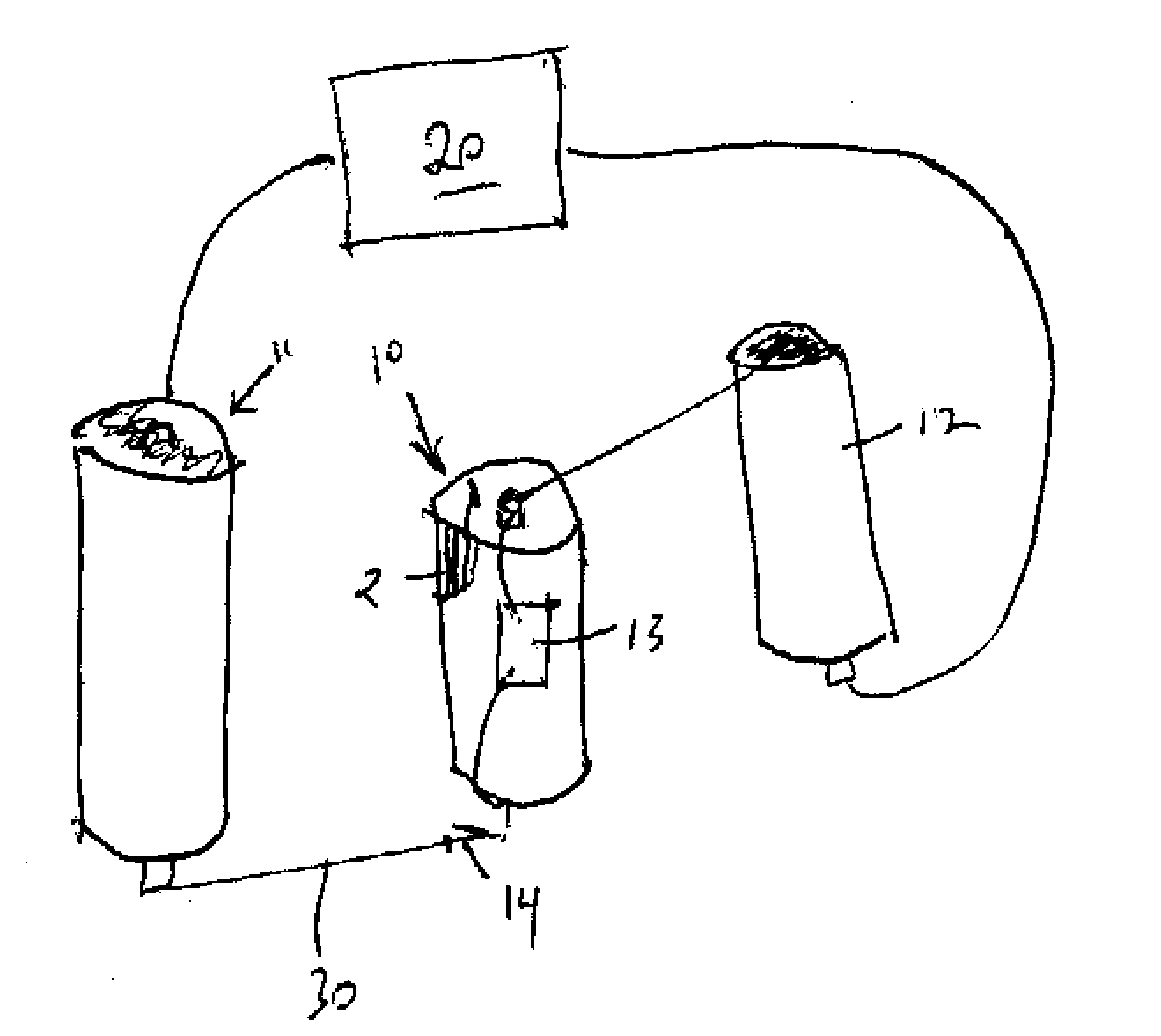
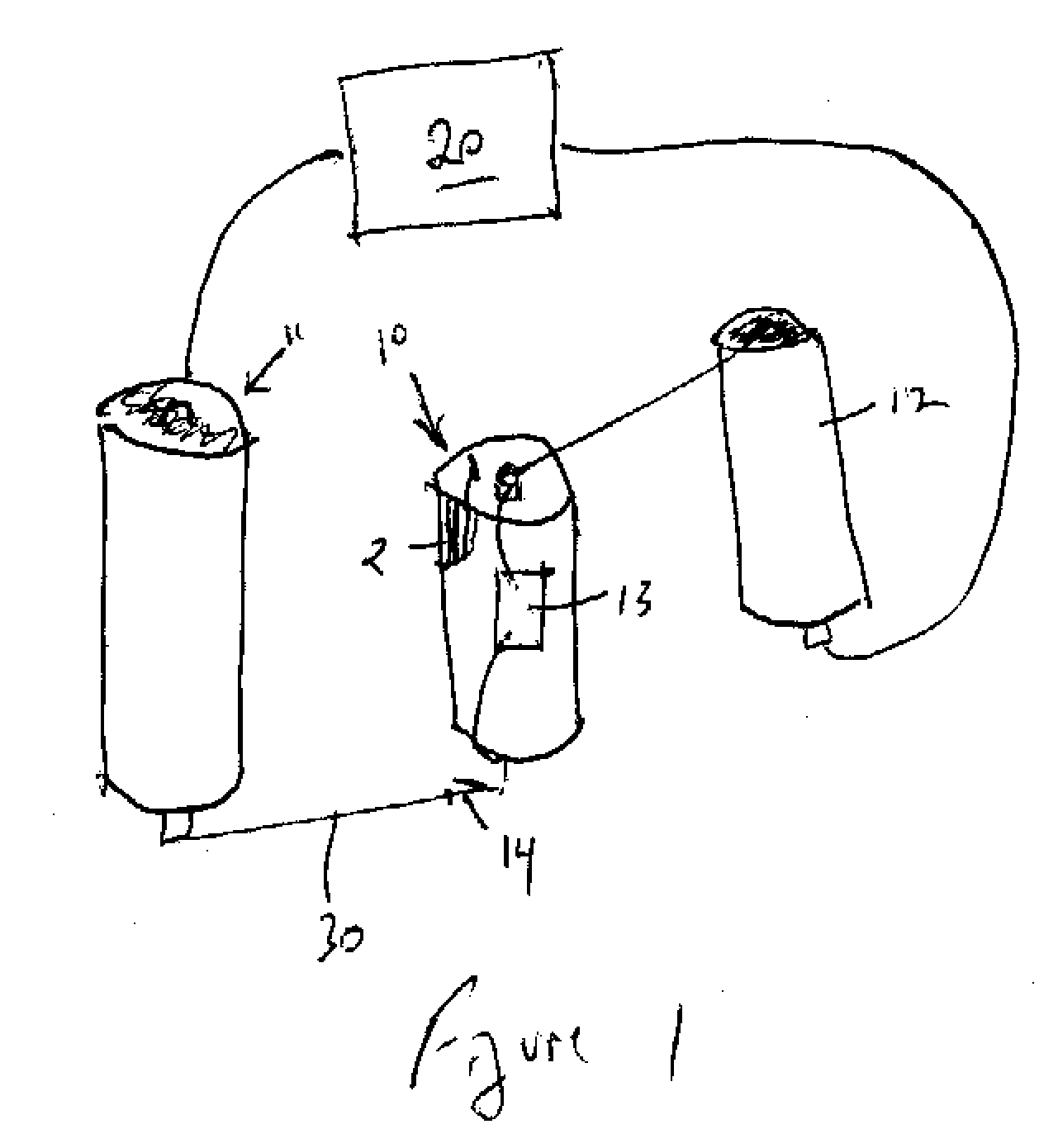
High discharge capacity lithium battery
InactiveUS20050233214A1Improve discharge performanceIncrease energy densityFinal product manufactureOrganic electrolyte cellsHigh rateIron disulfide
A lithium / iron disulfide electrochemical battery cell with a high discharge capacity. The cell has a lithium negative electrode, an iron disulfide positive electrode and a nonaqueous electrolyte. The iron disulfide of the positive electrode has a controlled average particle size range which allows the electrochemical cells to exhibit desired properties in both low and high rate applications. In various embodiments, the iron disulfide particles are wet milled, preferably utilizing a media mill or milled utilizing a non-mechanical mill such as a jet mill, which reduces the iron disulfide particles to a desired average particle size range for incorporation into the positive electrode.
Owner:EVEREADY BATTERY CO INC
Lithium cell
ActiveUS20090148756A1High affinityGood cohesive propertyElectrode rolling/calenderingSmall-sized cells cases/jacketsOrganic solventIron disulfide
A primary electrochemical cell having an anode comprising lithium and a cathode comprising iron disulfide (FeS2) and carbon particles. The cell is balanced so that the anode is in theoretical capacity excess (mAmp-hrs) compared to the theoretical capacity of the cathode. The anode and cathode can be spirally wound with separator therebetween and inserted into the cell casing with electrolyte then added. The electrolyte comprises a lithium salt dissolved in organic solvent.
Owner:DURACELL U S OPERATIONS
Method to make nickel positive electrodes and batteries using same
InactiveUS20010012586A1Large capacityImprove high temperature performanceElectrode manufacturing processesAlkaline accumulator electrodesAlkaline earth metalConductive polymer
This invention discloses a method to make a positive electrode and the nickel hydride battery using same. The positive electrode at least comprises a nickel hydroxide plus 1-15 wt. % of fine additive powders selected from the group consisting of Co / CoO, Ni, Cu, Zn, ZnO, C, Mg, Al, Mn, silver oxide, hydride, conductive polymer, and combinations thereof. Said positive electrode further comprises one, two or more additives, 0.01-10 wt. %, selected from the group of MgCl2, CaCl2, SrCl2, SrF2, BaCl2, BaF2, MgF2, and other fluorides / chlorides of alkali metals, alkaline earth metals, Al, Y, Sn, Sb, Ag, transition metals, rare earth metals, and composite metal oxide / halide to improve the performance of said positive electrode at high temperature.
Owner:HONG KUOCHIH +1
High discharge capacity lithium battery
A lithium / iron disulfide electrochemical battery cell with a high discharge capacity. The cell has a lithium negative electrode, an iron disulfide positive electrode and a nonaqueous electrolyte. The iron disulfide of the positive electrode has a controlled average particle size range which allows the electrochemical cells to exhibit desired properties in both low and high rate applications. In various embodiments, the iron disulfide particles are wet milled, preferably utilizing a media mill or milled utilizing a non-mechanical mill such as a jet mill, which reduces the iron disulfide particles to a desired average particle size range for incorporation into the positive electrode.
Owner:ENERGIZER BRANDS
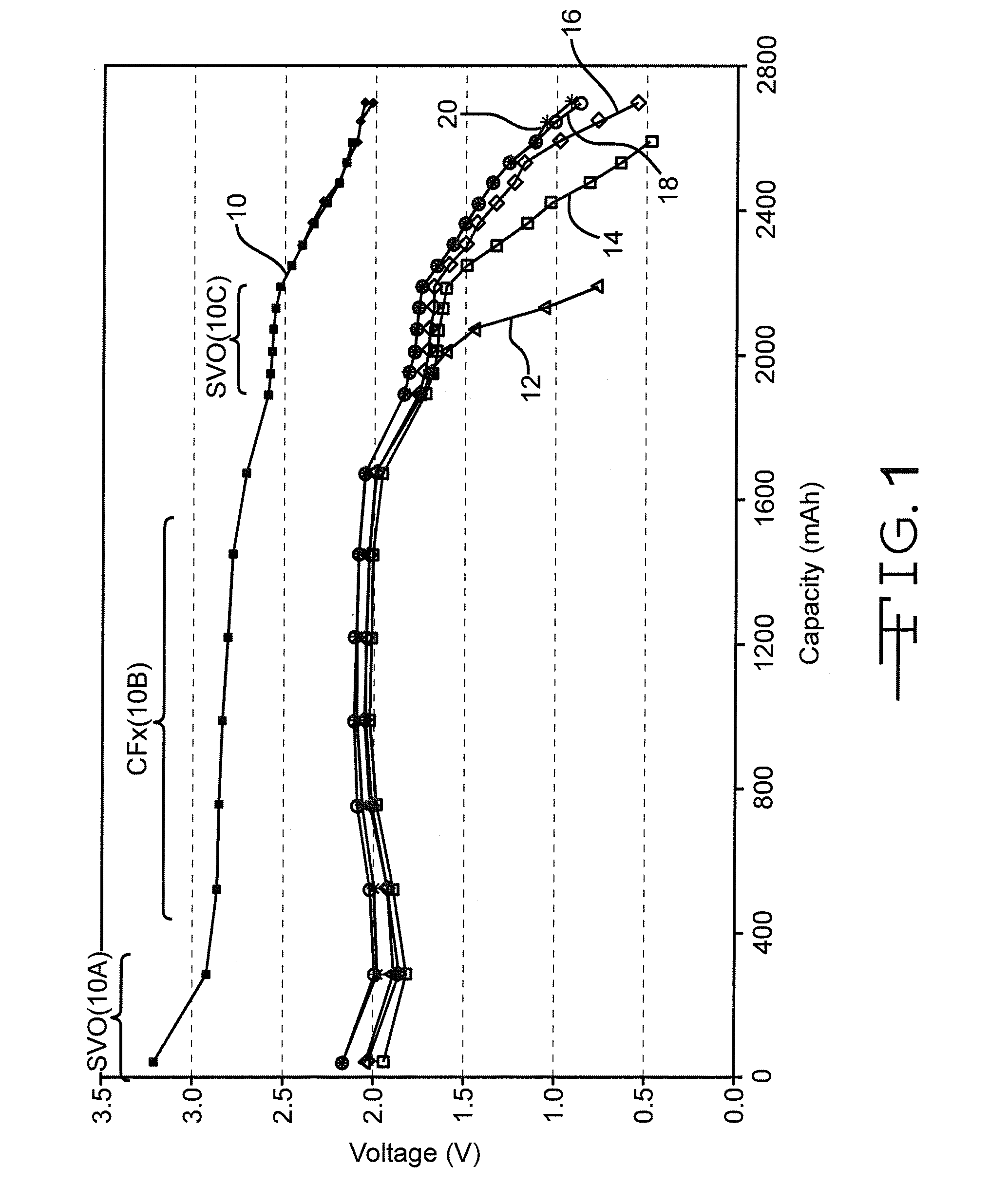
ANODE-TO-ANODE CAPACITY RATIOS FOR SVO/CFx HYBRID CATHODE ELECTROCHEMICAL CELLS
ActiveUS20070072075A1Improve performanceIncrease energy densitySilver accumulatorsElectrode carriers/collectorsHigh rateHigh energy
Improvements in the performance of lithium electrochemical cells comprising a first cathode active material of a relatively high energy density but of a relatively low rate capability, for example CFx, contacted to one side of a current collector and with a second cathode active material having a relatively low energy density but of a relatively high rate capability, for example SVO, contacted to the opposite current collector side are described. An exemplary cathode has the configuration: SVO / first current collector / CFx / second current collector / SVO, and wherein the anodic coulombic capacity does not exceed the total coulombic capacities of the SVO and CFx by greater than 25%. Manganese oxide (MnO2) is another typically used cathode active material in lieu of SVO, and the present invention is applicable to lithium cells of that system as well.
Owner:WILSON GREATBATCH LTD
Lithium-Iron Disulfide Cell Design
ActiveUS20100310910A1MiniaturizationEasy to controlCell seperators/membranes/diaphragms/spacersFinal product manufactureCell designOptoelectronics
A lithium-iron disulfide electrochemical cell design is disclosed, relying on judicious selection of the electrolyte, a thicker lithium anode and a cathode with specific characteristics selected to cooperate with the electrolyte. The resulting cell has a reduced interfacial surface area between the anode and the cathode but, surprisingly, maintains excellent high drain rate capacity.
Owner:ENERGIZER BRANDS
High discharge capacity lithium battery
ActiveCN1883065ACell seperators/membranes/diaphragms/spacersSmall-sized cells cases/jacketsHigh concentrationHigh rate
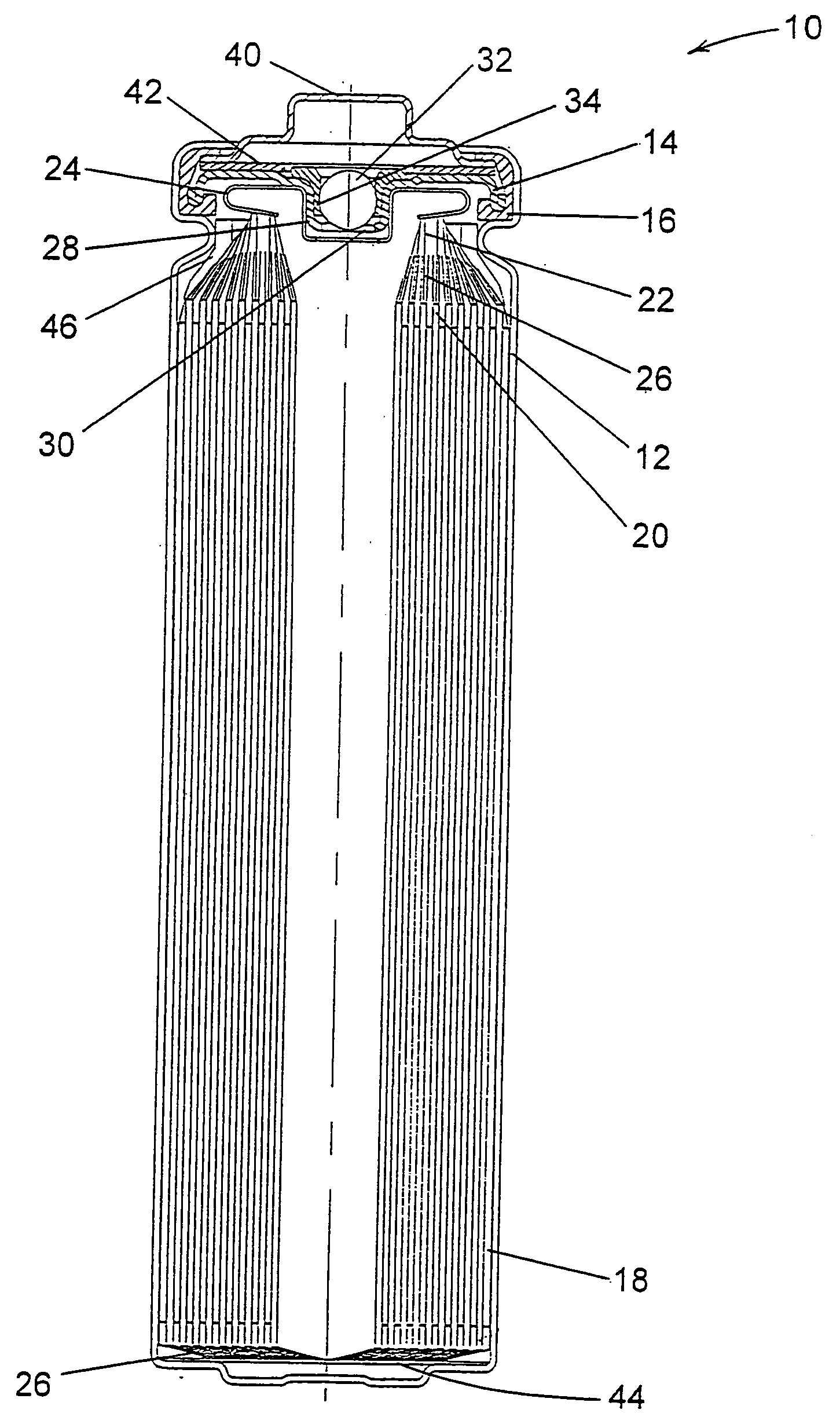
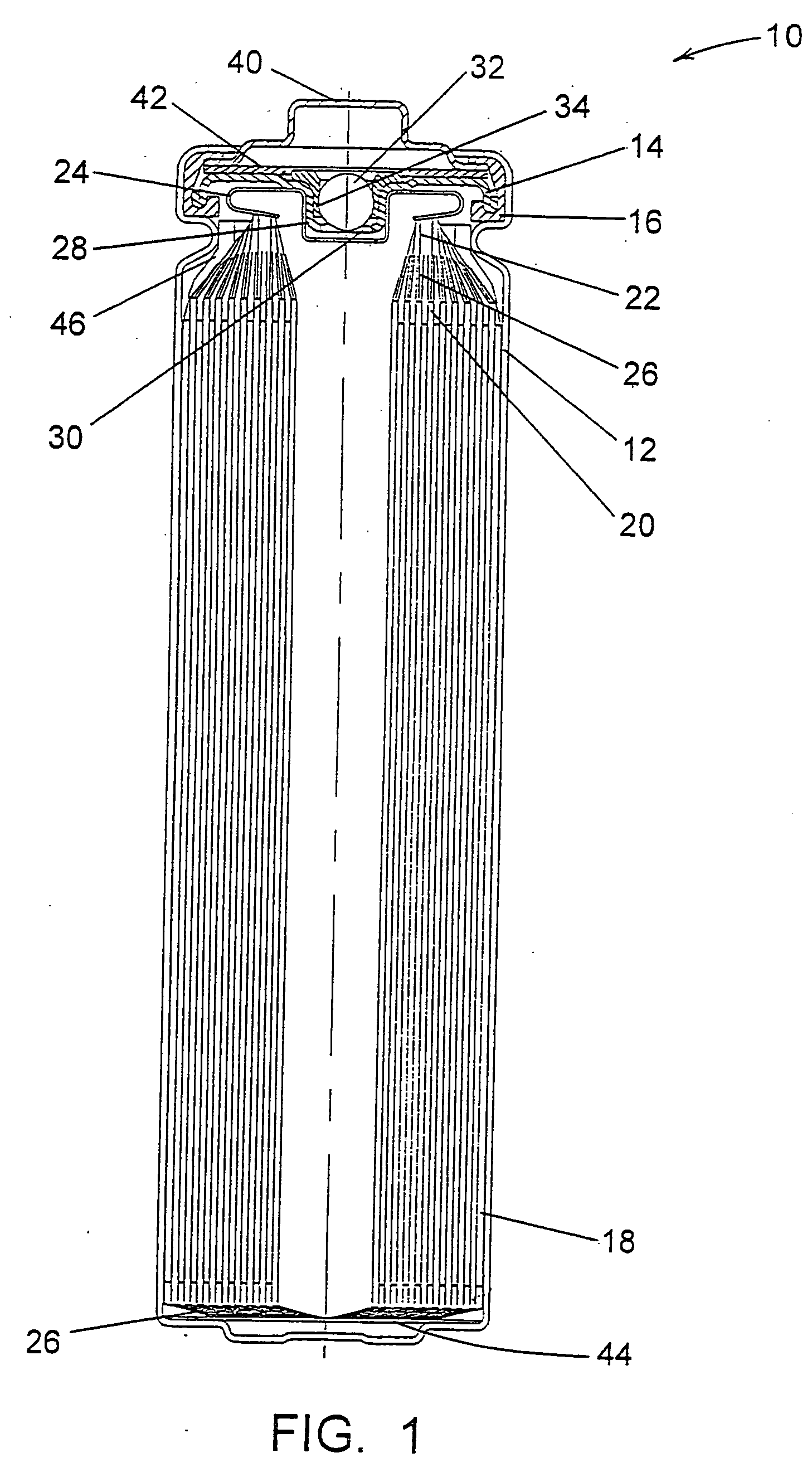
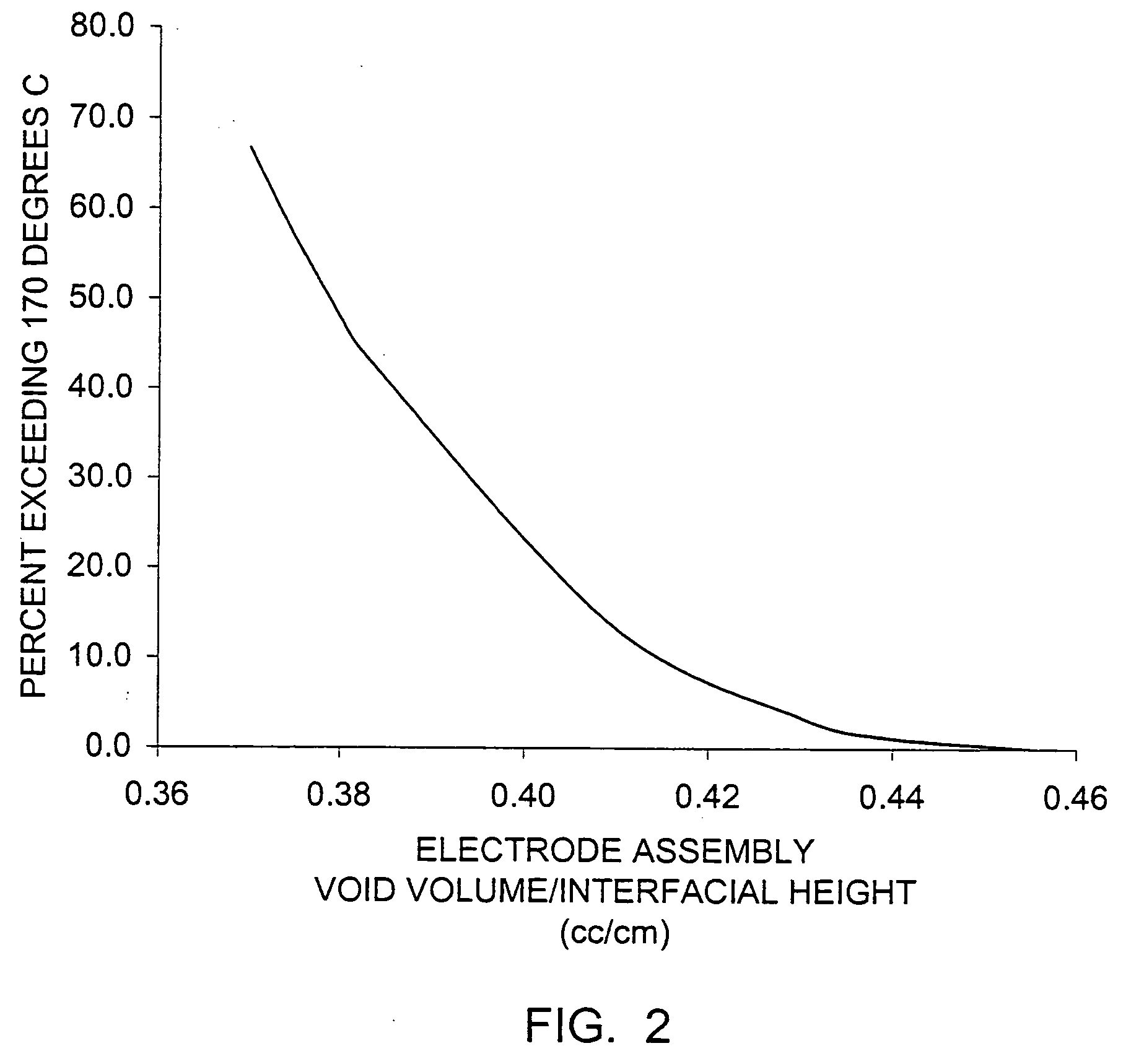
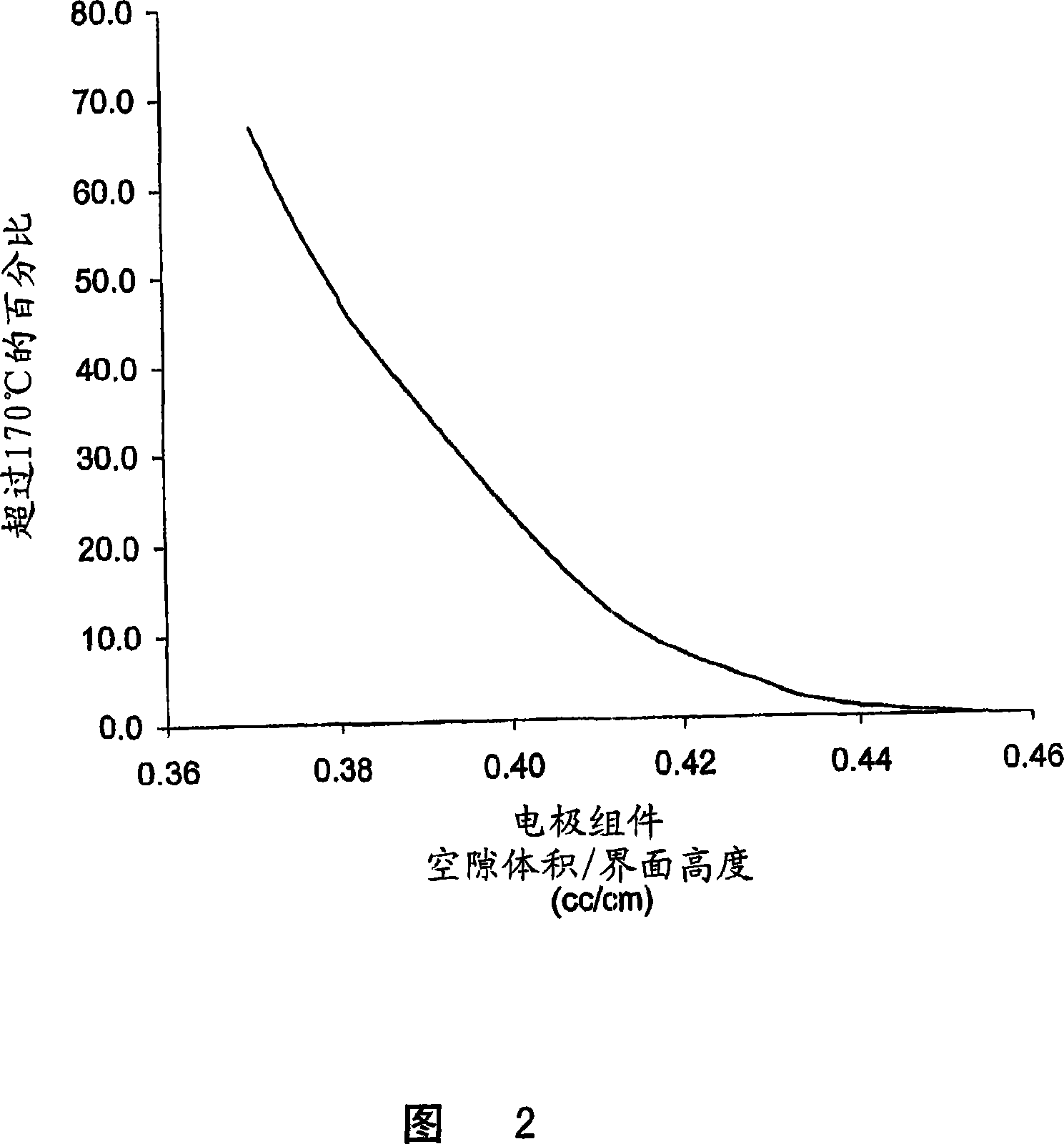
A lithium / iron disulfide electrochemical battery cell with a high discharge capacity. The cell has a lithium negative electrode, an iron disulfide positive electrode and a nonaqueous electrolyte. The positive electrode mixture containing the iron disulfide contains highly packed solid materials, with little space around the solid particles, to provide a high concentration of iron disulfide within the mixture. The separator is thin, to allow more space within the cell for active materials, yet strong enough to prevent short circuits between the positive and negative electrodes under abusive conditions, even when swelling of the cathode during cell discharge places additional stressed on the separator. As a result, the ratio of the interfacial capacity of the positive electrode to the electrode interfacial volume is high, as is the actual capacity on low rate / low power and high rate / high power discharge.
Owner:ENERGIZER BRANDS
Method For Coating Noble Metals On Titanium Current Collectors For Use In Nonaqueous Li/CFx Cells
InactiveUS20060141340A1Improve simplicityElectrode manufacturing processesFinal product manufactureIridiumCarbon coating
A lithium / fluorinated carbon electrochemical cell having the CFx material supported on a titanium current collector screen sputter coated with a noble metal is described. The gold, iridium, palladium, platinum, rhodium and ruthenium-coated titanium current collector provides the cell with higher rate capability, even after exposure to high temperatures, in comparison to cells of a similar chemistry having the CFx contacted to a titanium current collector painted with a carbon coating.
Owner:WILSON GREATBATCH LTD
SVO/CFx parallel cell design within the same casing
InactiveUS6926991B2Improve performanceImprove capacity efficiencySilver accumulatorsElectrode thermal treatmentHigh rateHigh energy
A new cathode design has a first cathode active material of a relatively low energy density but of a relatively high rate capability contacted to a first cathode current collector and a second cathode active material having a relatively high energy density but of a relatively low rate capability in contact with a second cathode current collector, is described. The first and second cathode current collectors are connected to a common terminal lead. The present cathode design is useful for powering an implantable medical device requiring a high rate discharge application.
Owner:WILSON GREATBATCH LTD
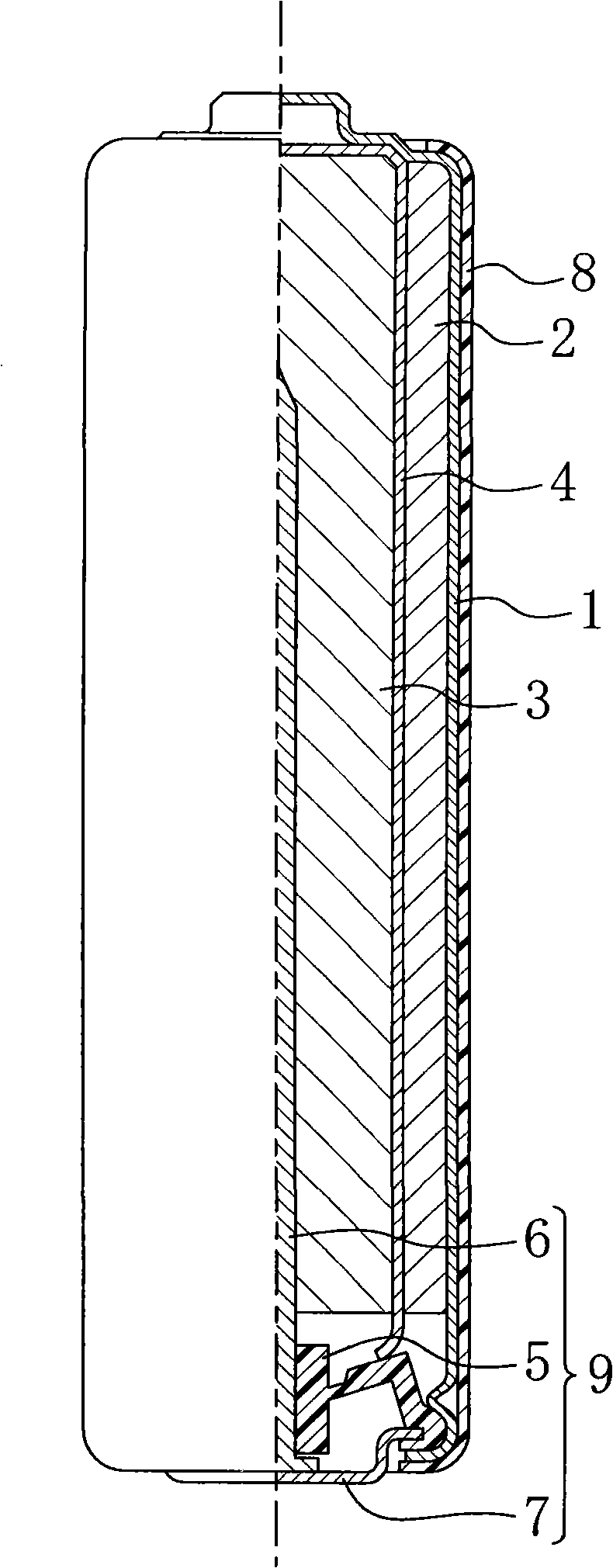
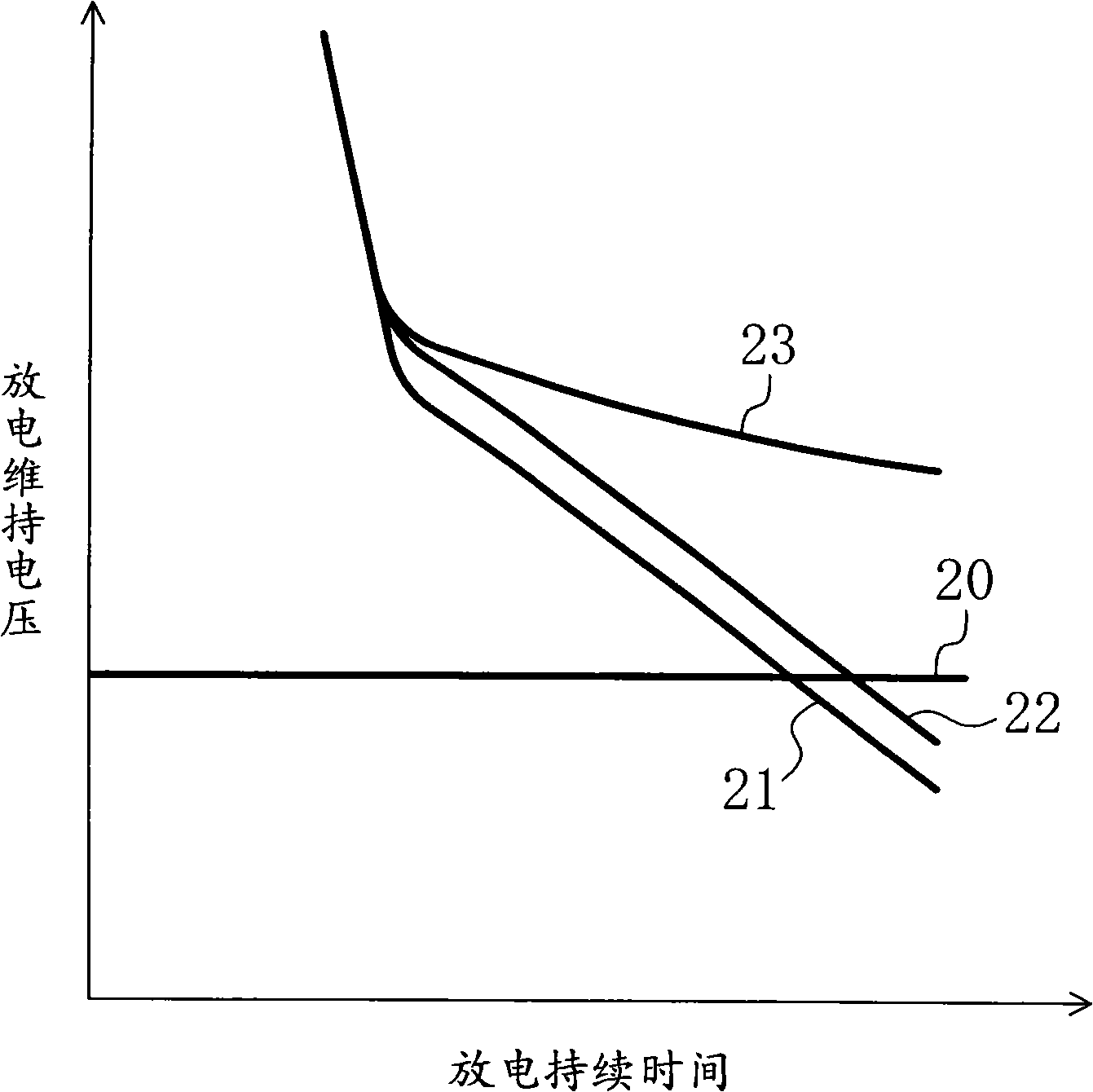
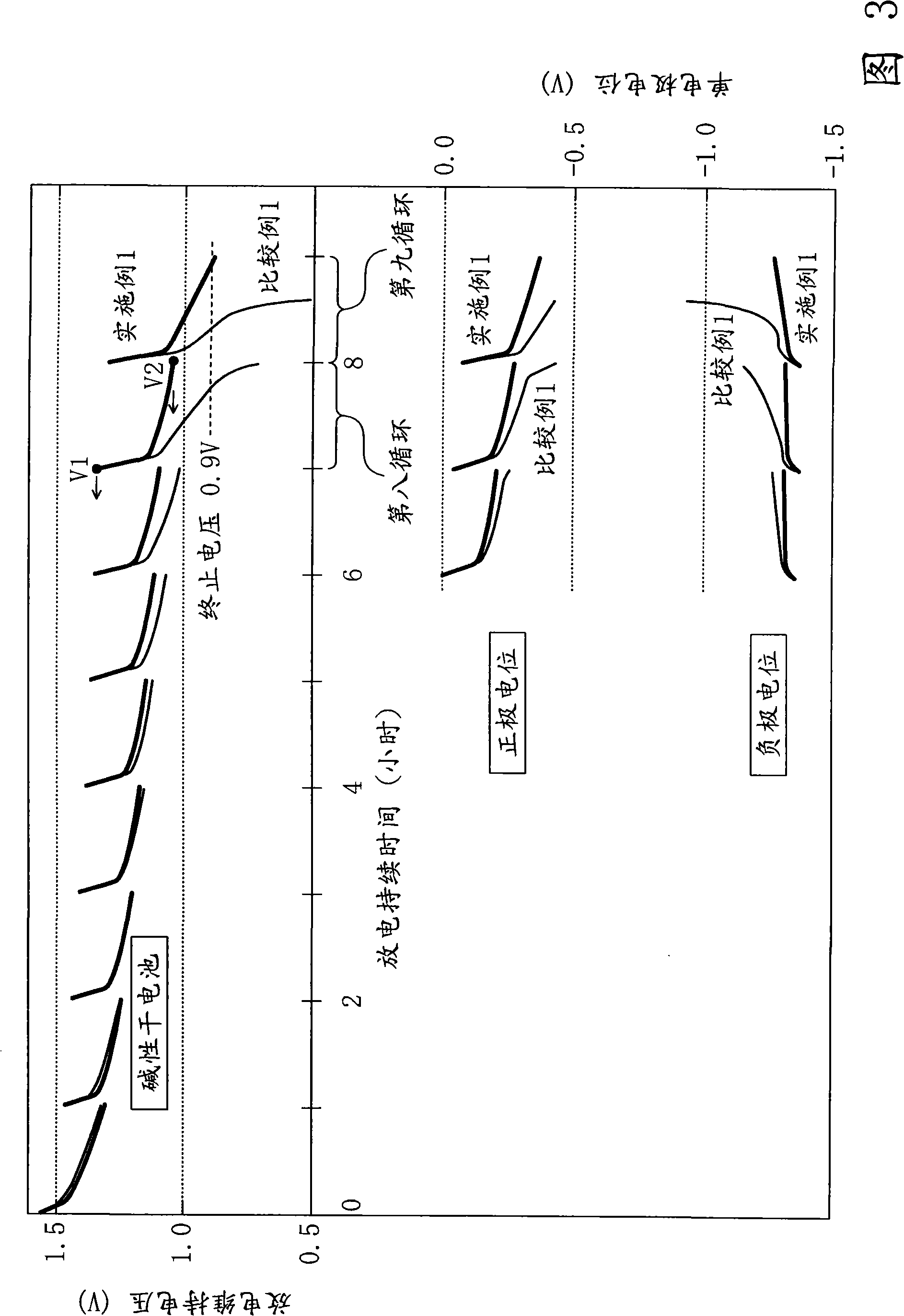
Battery
ActiveUS20070218357A1Improve load characteristicsIncrease discharge voltageOrganic electrolyte cellsSecondary cell gas removalLithium metalEngineering
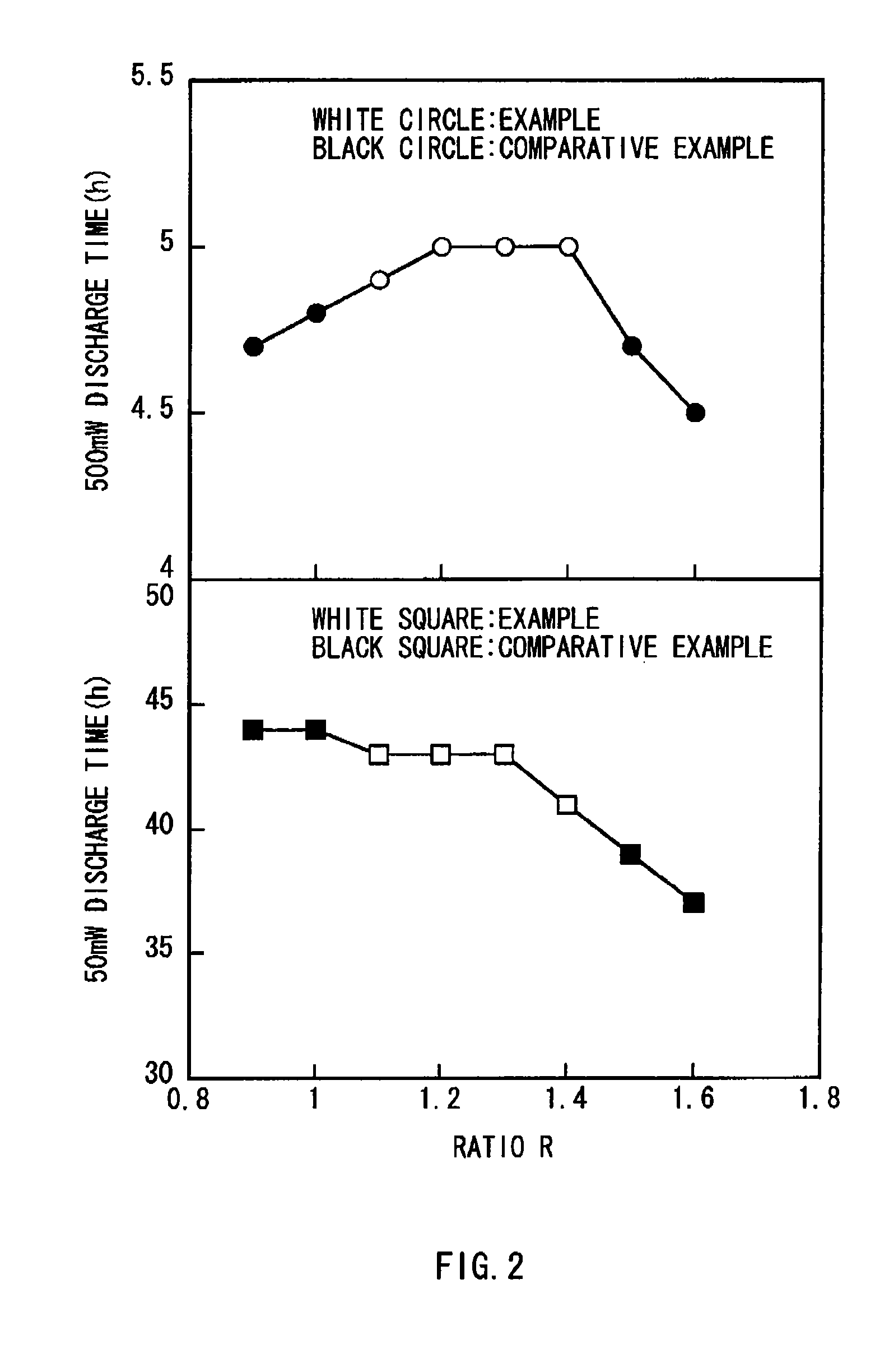
A battery capable of improving the constant output discharge capacity is provided. A battery includes a cathode, an anode, and an electrolyte. The cathode contains iron sulfide. The anode contains lithium metal or a lithium alloy. A ratio of a discharge capacity per unit area of the cathode to a discharge capacity per unit area of the anode (the discharge capacity per unit area of the cathode / the discharge capacity per unit area of the anode) is more than 1 and 1.4 or less.
Owner:MURATA MFG CO LTD
Anticorrosive electrolyte of lithium battery and obtained lithium primary battery
InactiveCN106450365AImprove electrochemical performanceImprove securityOrganic electrolyte cellsCell preserving/storageElectrical batteryHeat stability
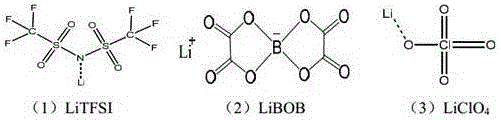
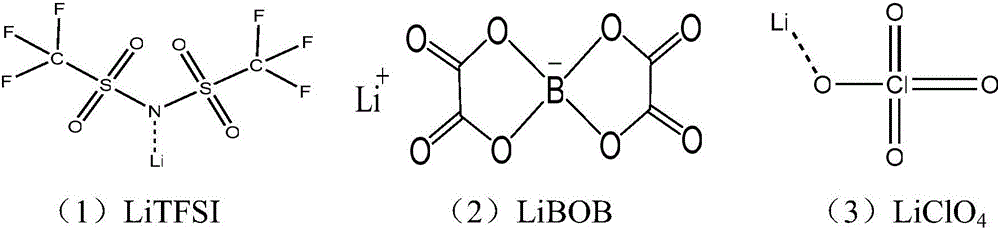
The invention belongs to the technical field of chemical batteries, and particularly relates to an anticorrosive electrolyte of a lithium battery and an obtained lithium primary battery. The anticorrosive electrolyte of the lithium battery comprises an organic solvent and an electrolyte, wherein the electrolyte is a mixed lithium salt; and the mixed lithium salt comprises lithium bis(trifluoromethanesulfonyl ) imide LiTFSI, lithium bis(oxalatoe ) borate LiBOB and lithium perchlorate. The lithium salt with a good anticorrosive effect and good heat stability is added to the electrolyte, so that the problems that aluminum foil is corroded and the battery is short in service life and poor in safety due to use of a high-conductivity fluorine-containing lithium salt are solved on the basis of not reducing the capacity of the battery and the protection effect on the aluminum foil. In addition, the electrolyte provided by the invention is suitable for the electrolytes, such as lithium / manganese dioxide (Li / MnO2), lithium / iron disulfide (Li / FeS2), lithium / copper oxide (Li / CuO), lithium / fluorinated carbon [Li / (CF)x], used for the lithium primary battery, and has the advantages of high applicability, a wide range and the like.
Owner:HUIZHOU HUIDERUI LITHIUM BATTERY TECHNOLOGY CO LTD
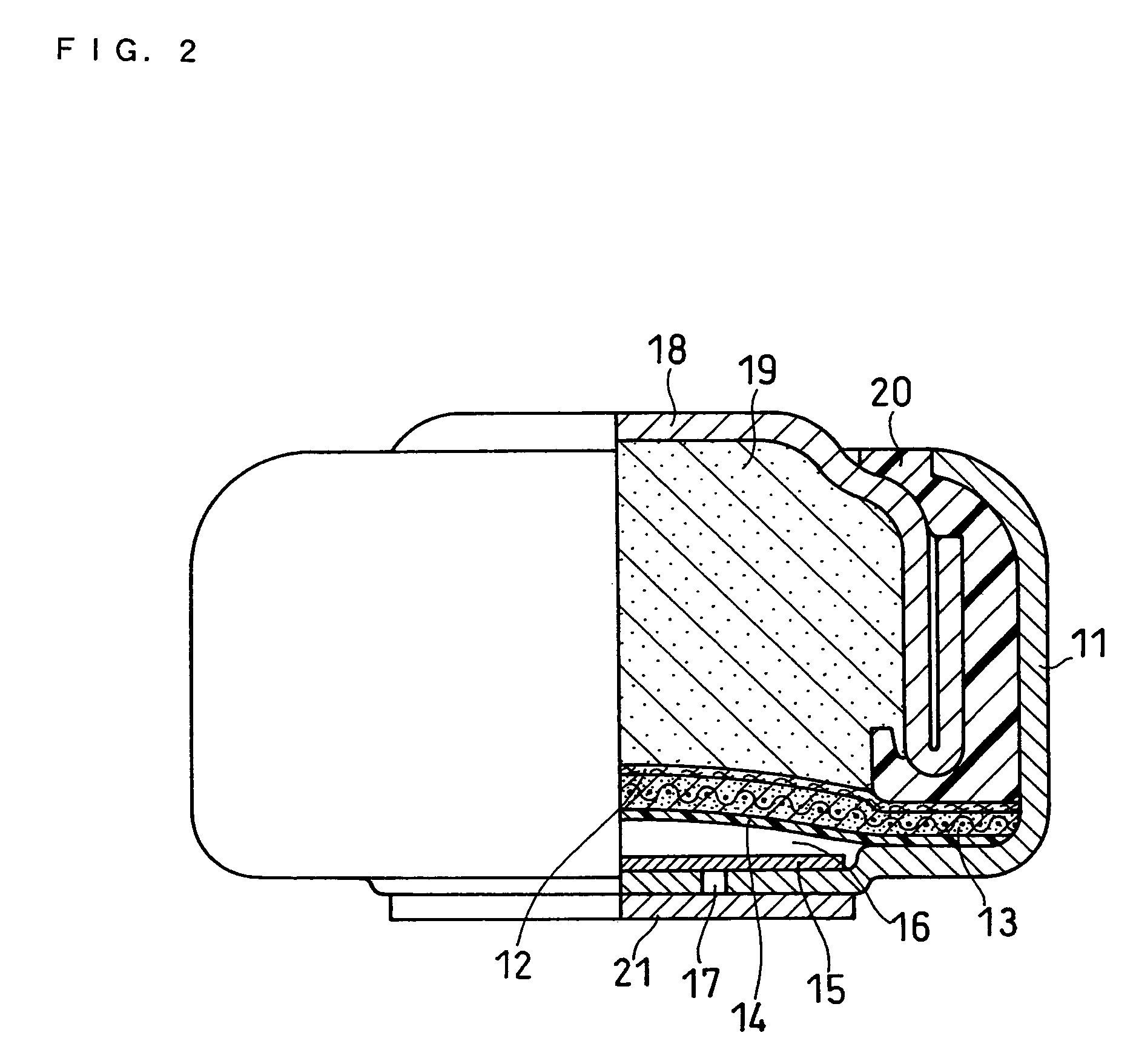
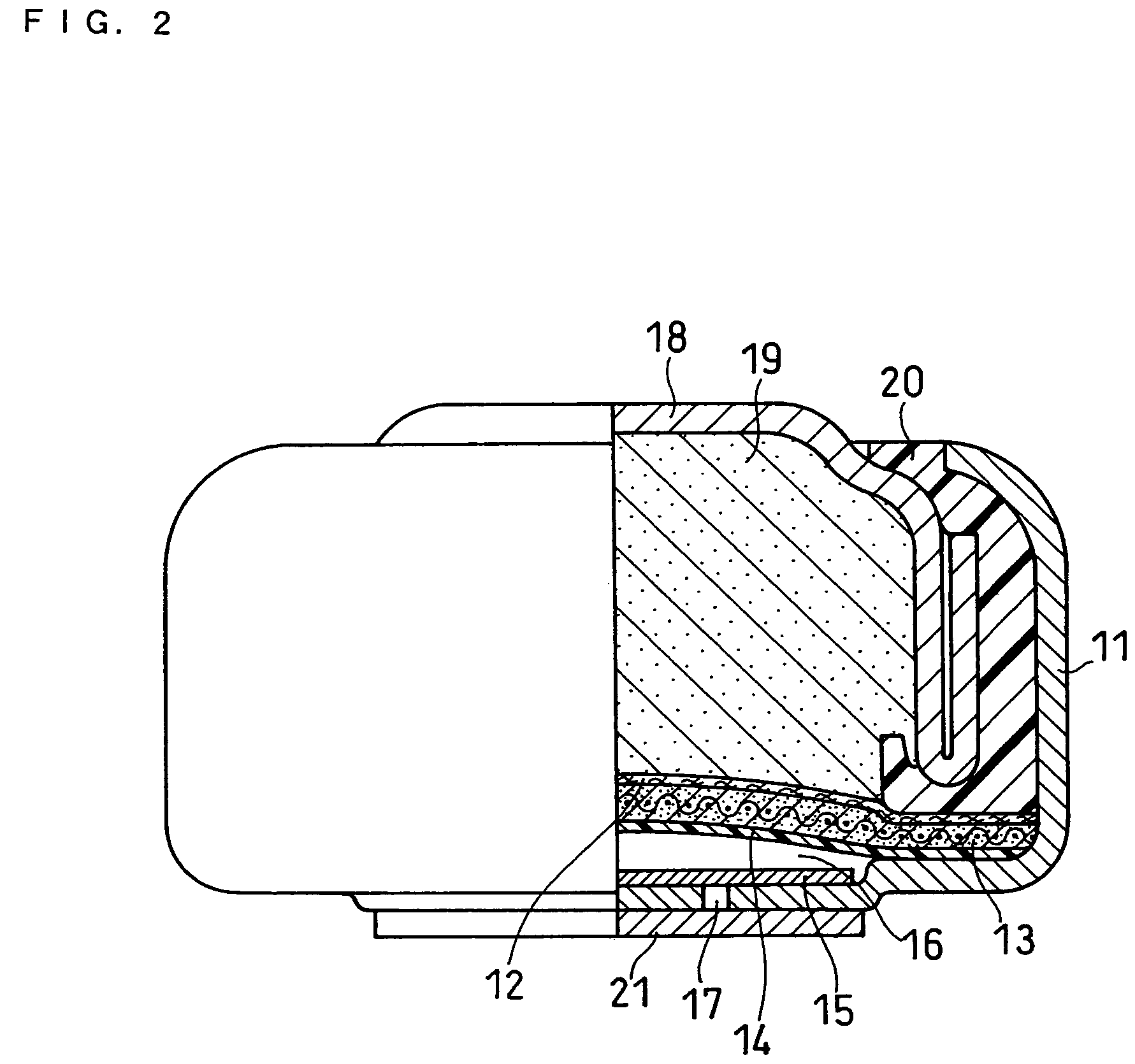
Alkaline cell with flat housing
InactiveUS7491464B2Improve performanceImprove utilizationClosuresFinal product manufactureHigh pressureBiomedical engineering
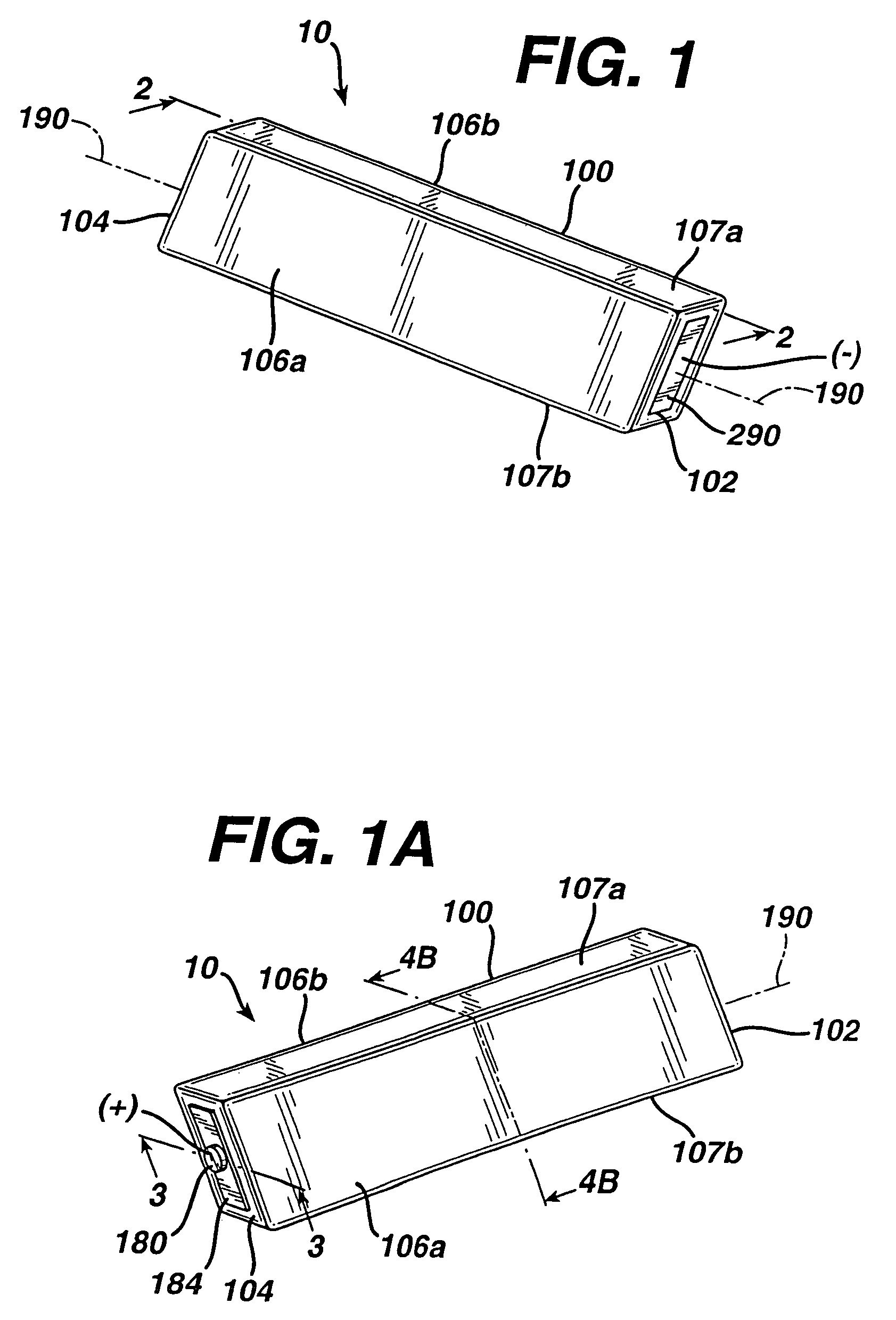
An alkaline cell having a flat housing, preferably of cuboid shape. The housing may be laser welded closed with a metal cover inserted as a part of an end cap assembly. A portion of an insulating seal may be held compressed between a sleeve extending from the metal cover and an anode current collector nail within the end cap assembly. The housing may include a vent mechanism, preferably a grooved vent, which can activate, when gas pressure within the cell reaches a threshold level typically between about 250 and 800 psig (1724×103 and 5515×103 pascal gage). The cell can have a supplemental laser welded vent which may activate at higher pressure levels.
Owner:DURACELL U S OPERATIONS
High discharge capacity lithium battery
Owner:ENERGIZER BRANDS
Alkaline battery
InactiveUS20070141466A1Excellent characteristicsIncrease resistanceFuel and primary cellsPrimary cell electrodesElectrolyte leakageHigh rate
An alkaline battery includes: a negative electrode including a zinc or zinc alloy powder as an active material; an alkaline electrolyte; and a positive electrode. The zinc or zinc alloy powder has a specific surface area of 0.01 to 10 m2 / g, and the weight ratio of the electrolyte to the negative electrode active material is in the range of 0.1 to 2. This invention can provide an alkaline battery that is improved in electrolyte leakage resistance and high-rate discharge characteristics.
Owner:PANASONIC CORP
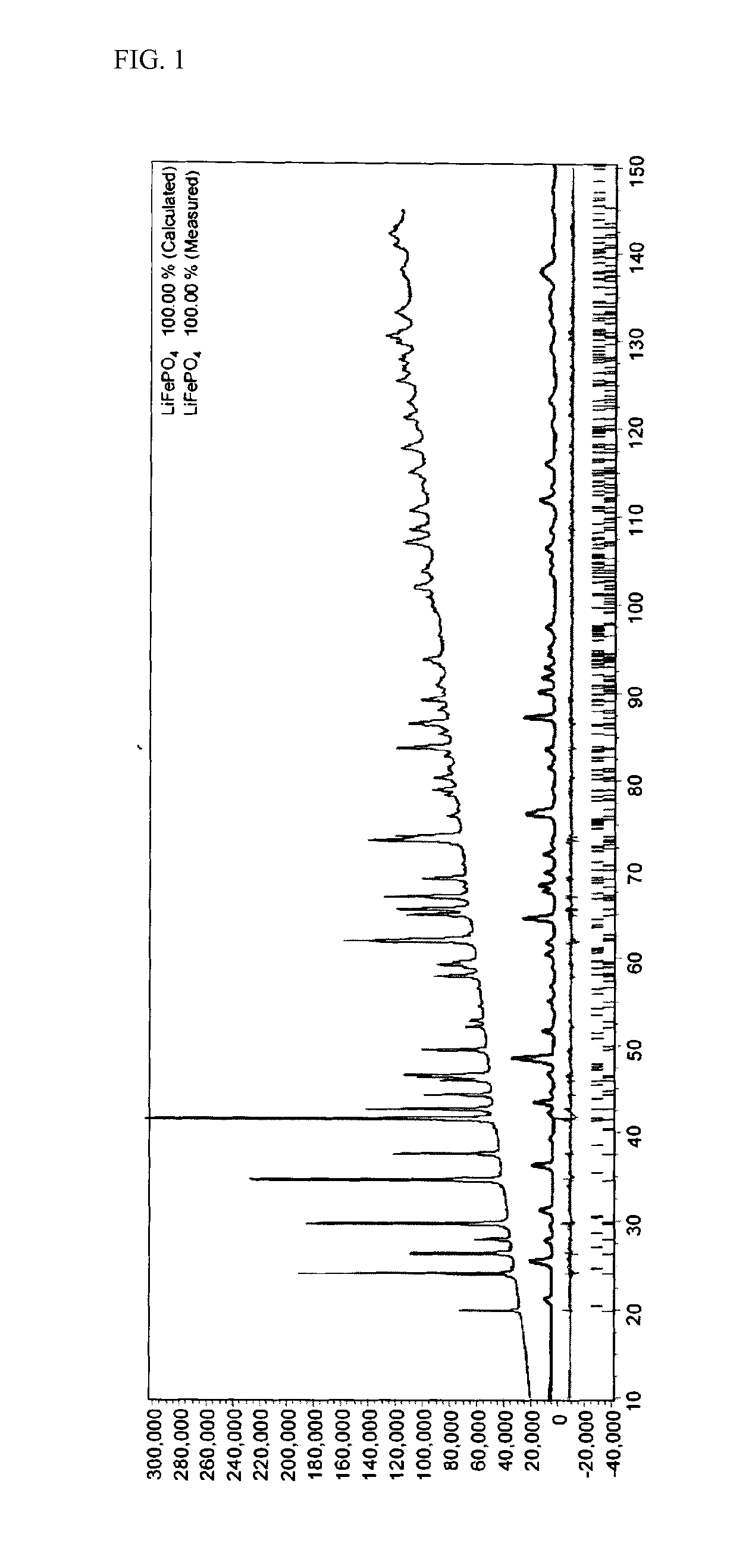
Cathode active material providing improved efficiency and energy density of electrode
ActiveUS20110287315A1Leveling of efficiencyReduced operating requirementsMaterial nanotechnologyElectrode manufacturing processesStructural deformationHigh energy
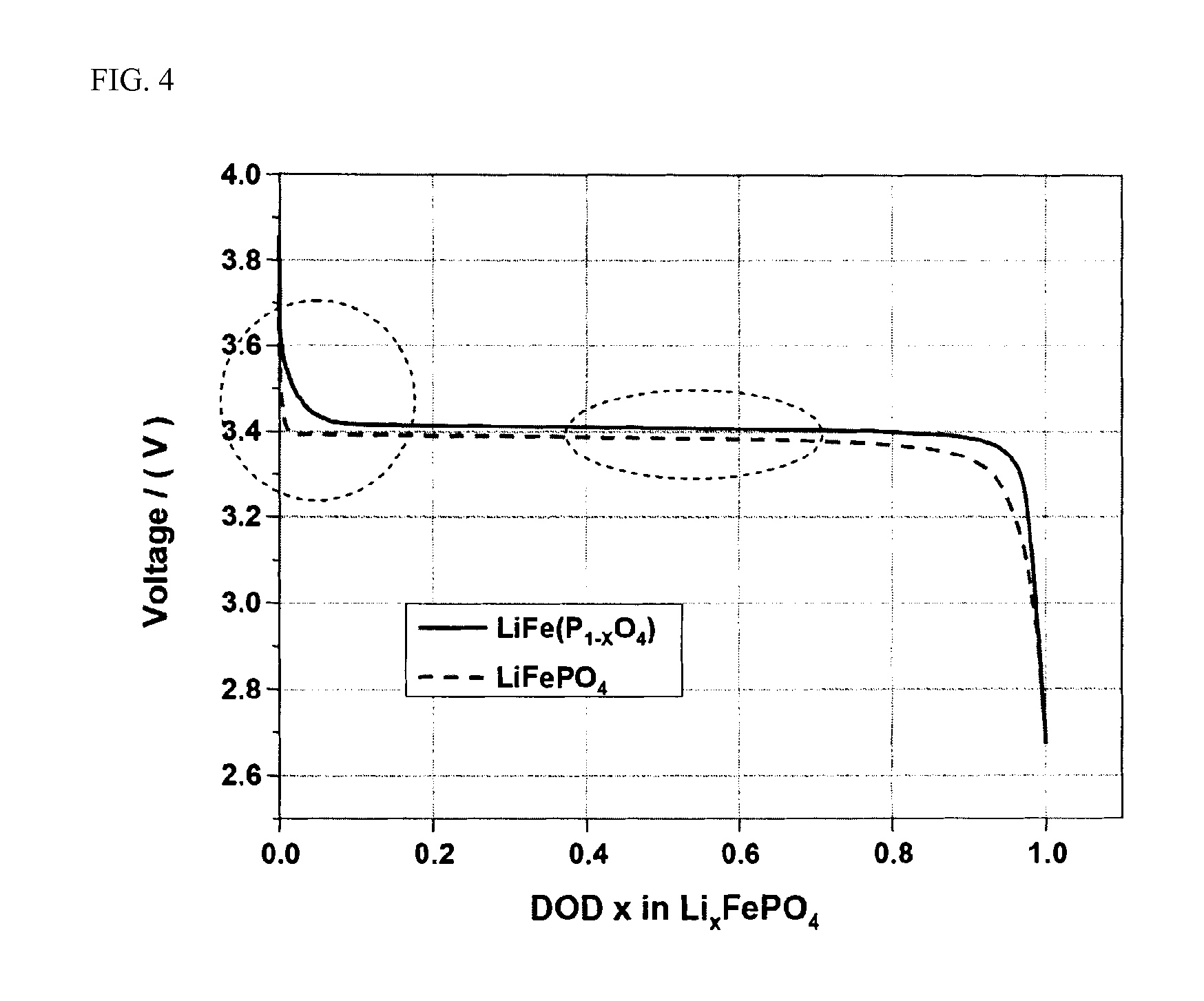
Provided is a cathode active material having a composition represented by the following Formula I: LiFe(P1-XO4) (I) wherein a molar fraction (1-x) of phosphorus (P) is in the range of 0.910 to 0.999, to allow operational efficiency of the cathode active material to be leveled to a lower operational efficiency of an anode active material and improve energy density of the cathode active material. Furthermore, a cathode active material, wherein a molar fraction (1-x) of phosphorus (P) is lower than 1, contains both Fe2+ and Fe3+, thus advantageously preventing structural deformation, improving ionic conductivity, exhibiting superior rate properties and inhibiting IR drop upon charge / discharge, thereby imparting high energy density to batteries.
Owner:LG ENERGY SOLUTION LTD
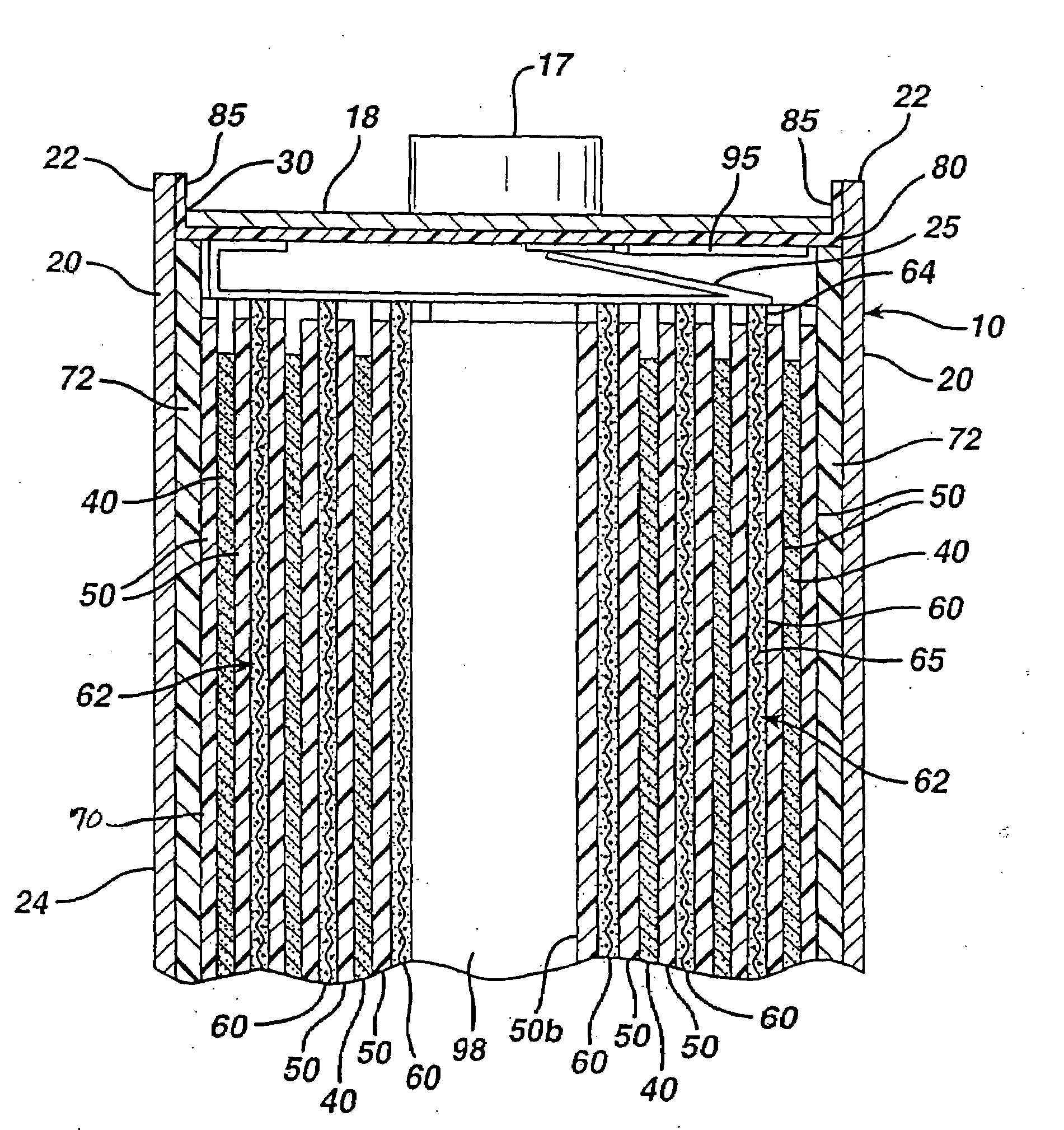
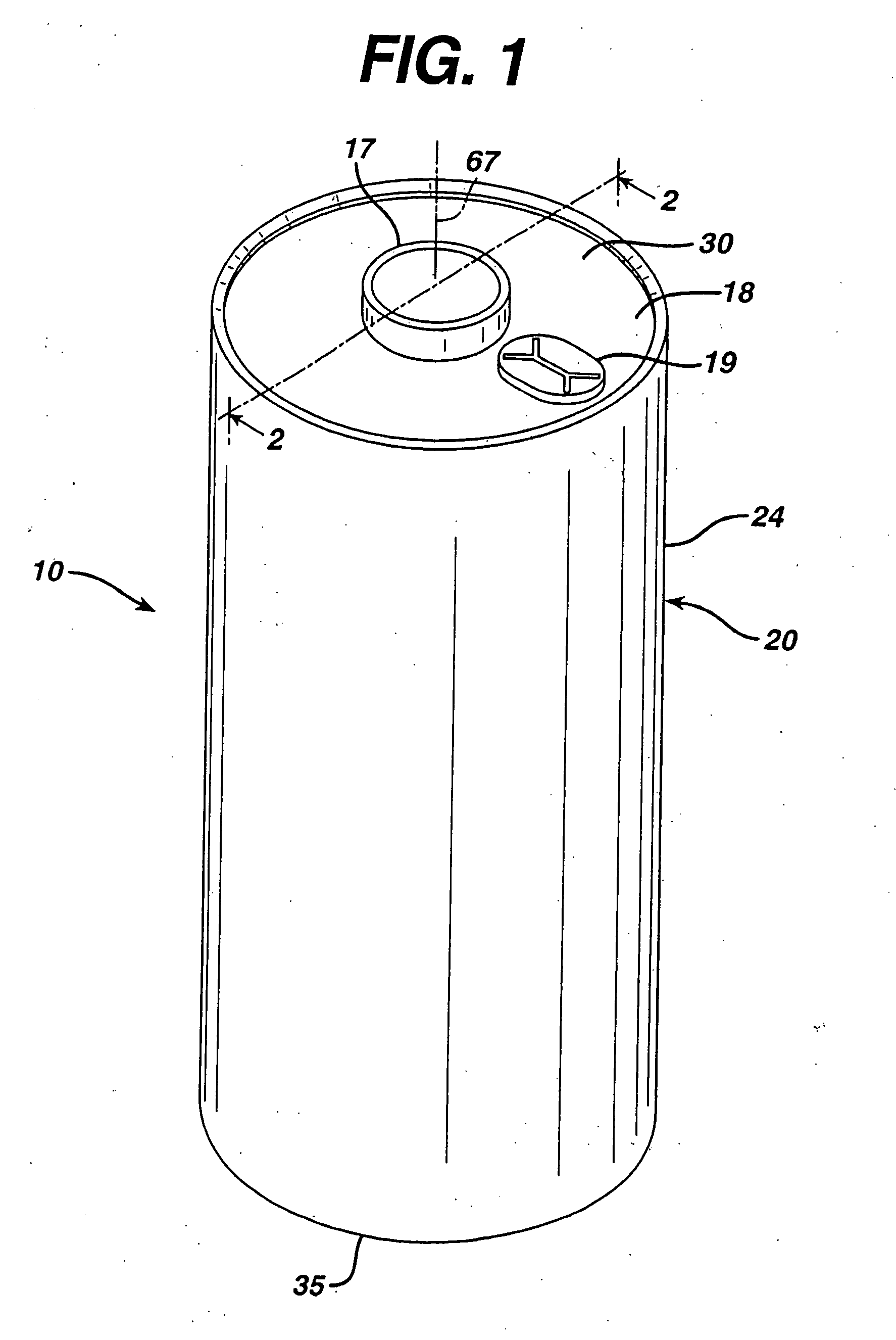
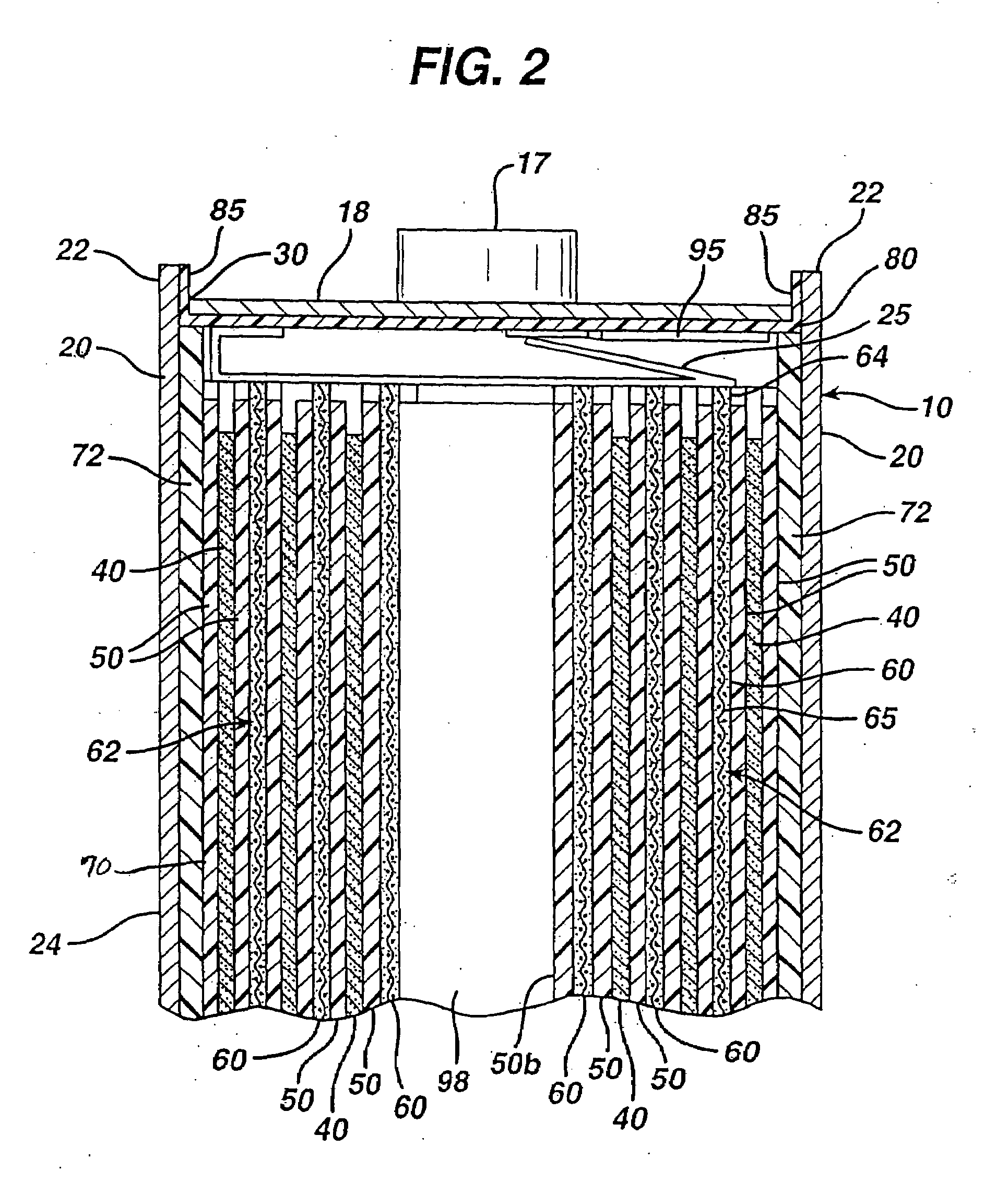
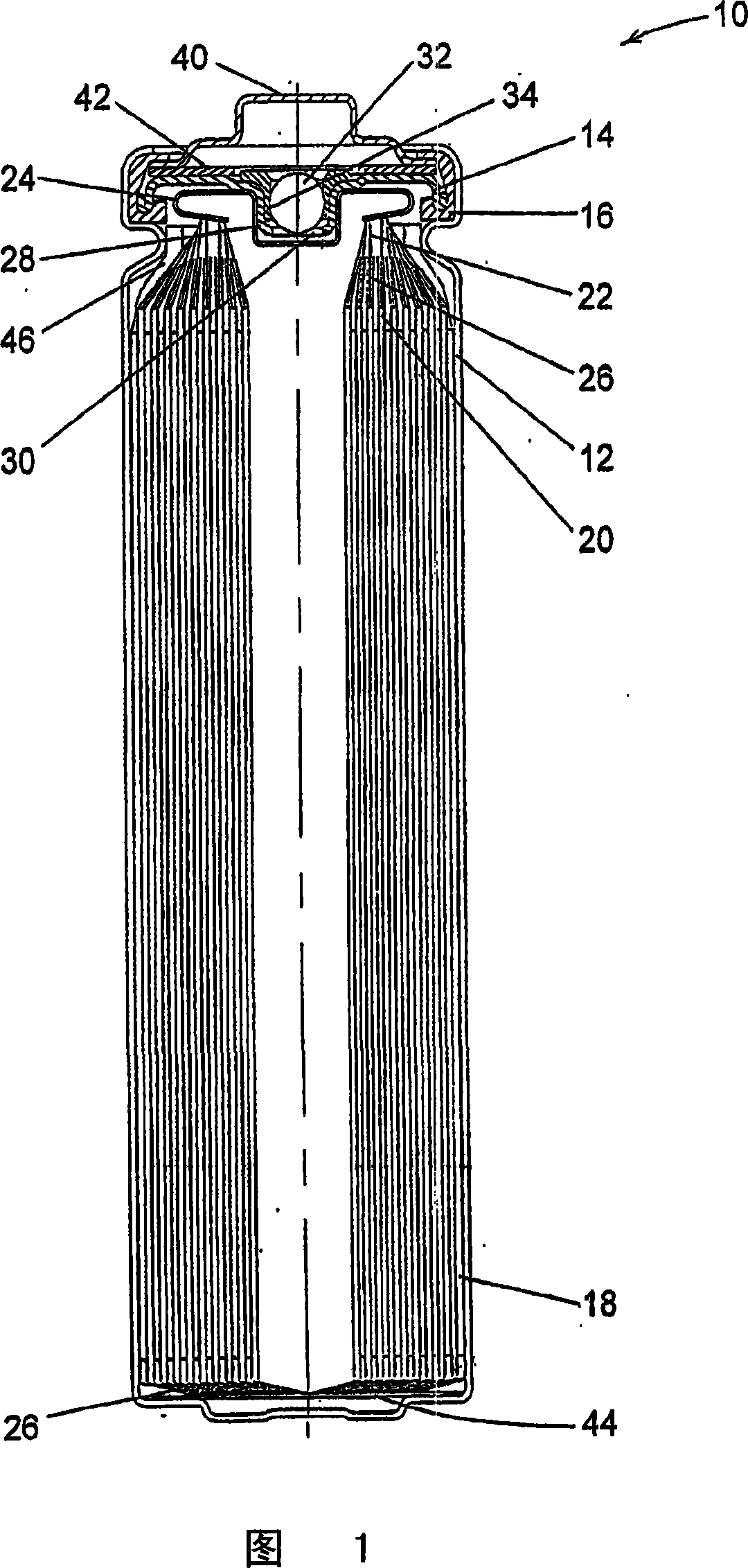
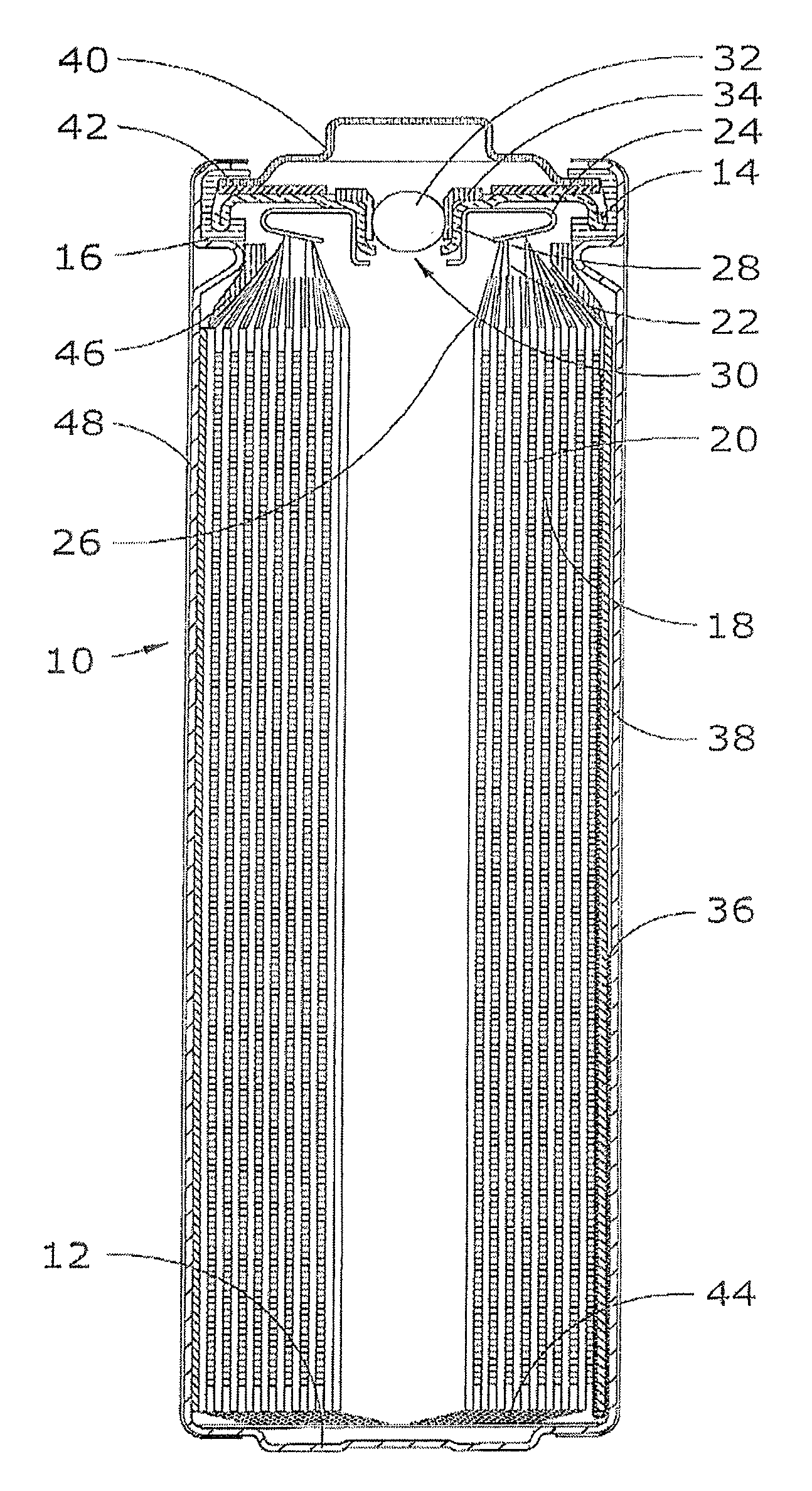
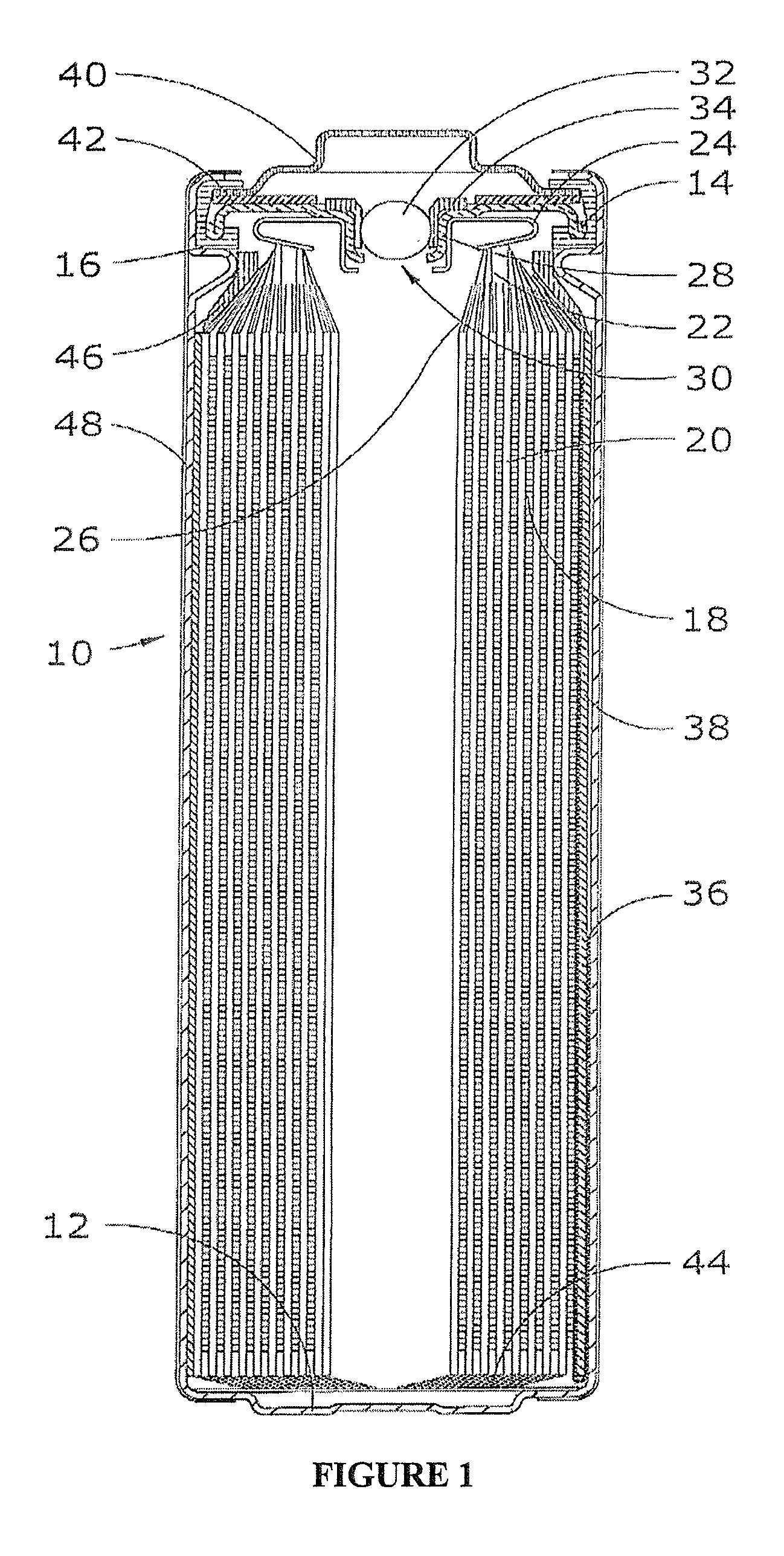
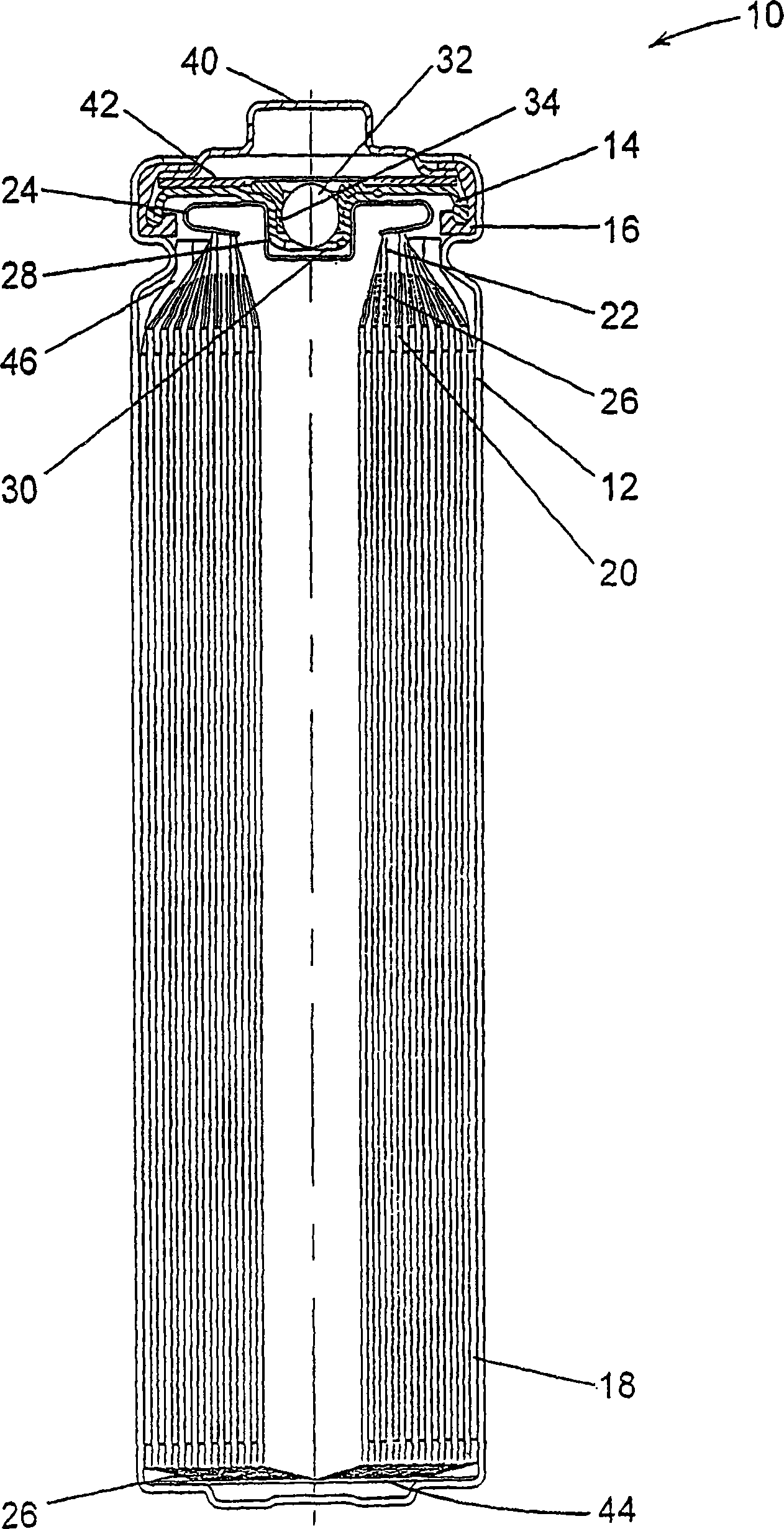
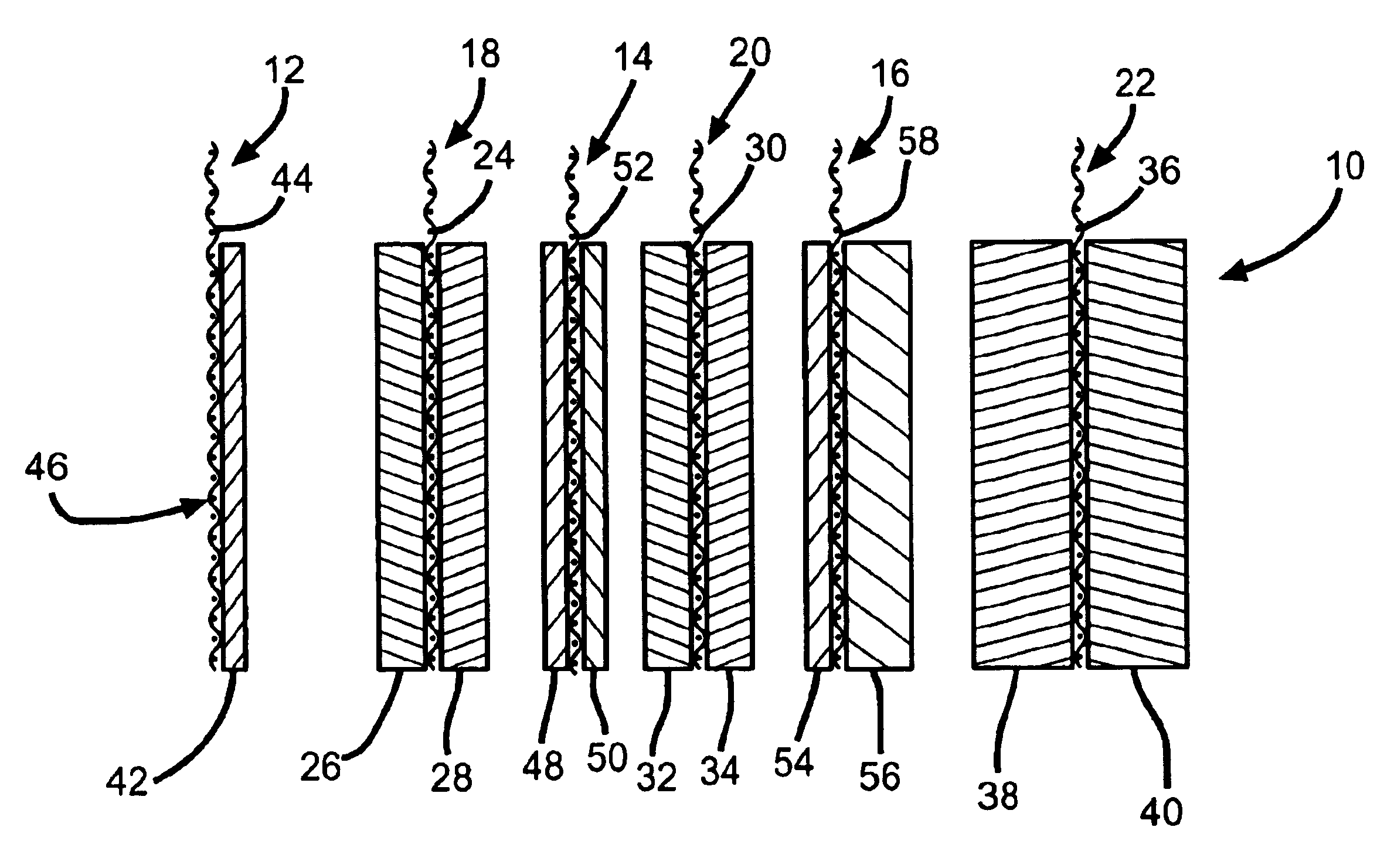
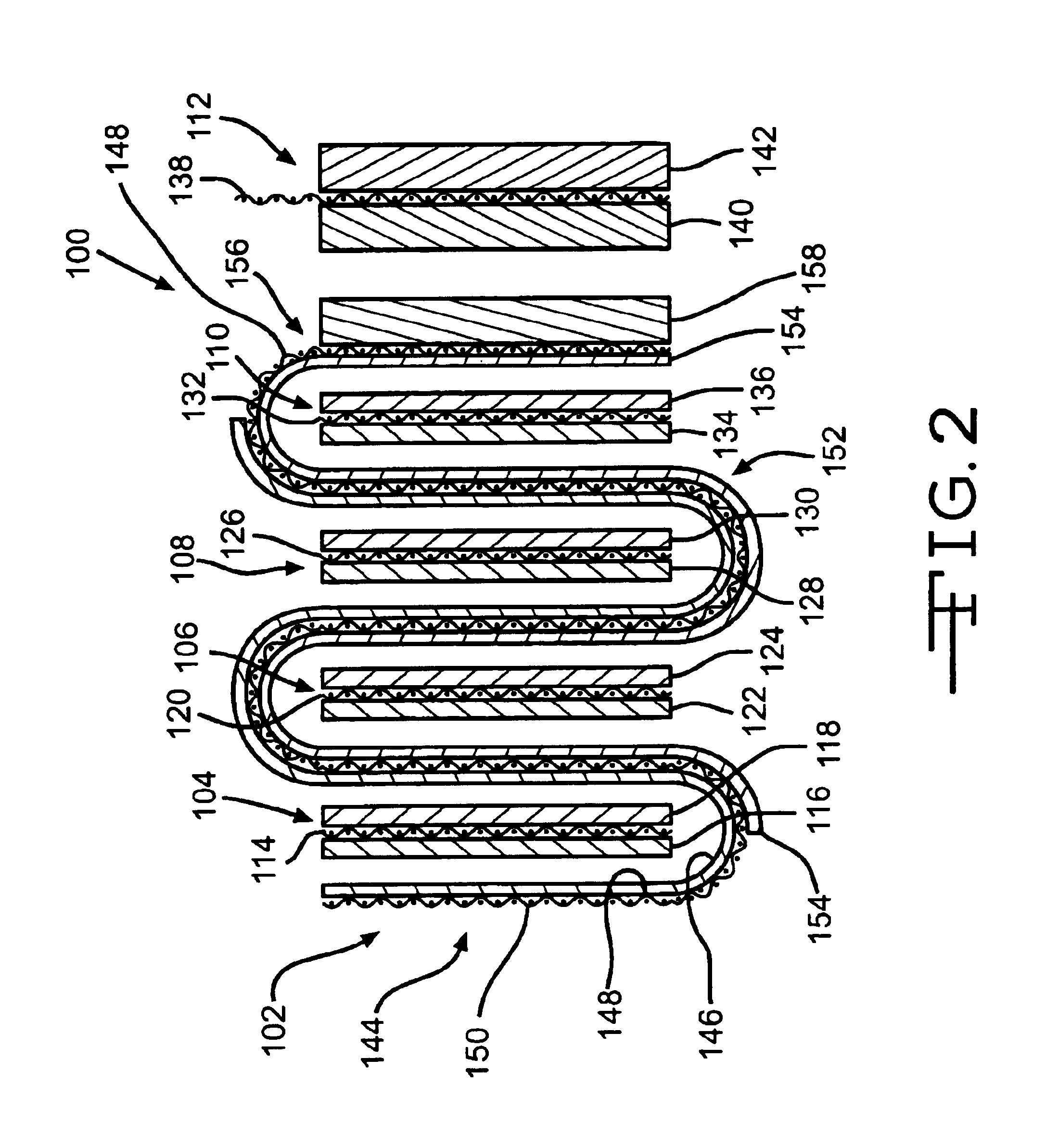
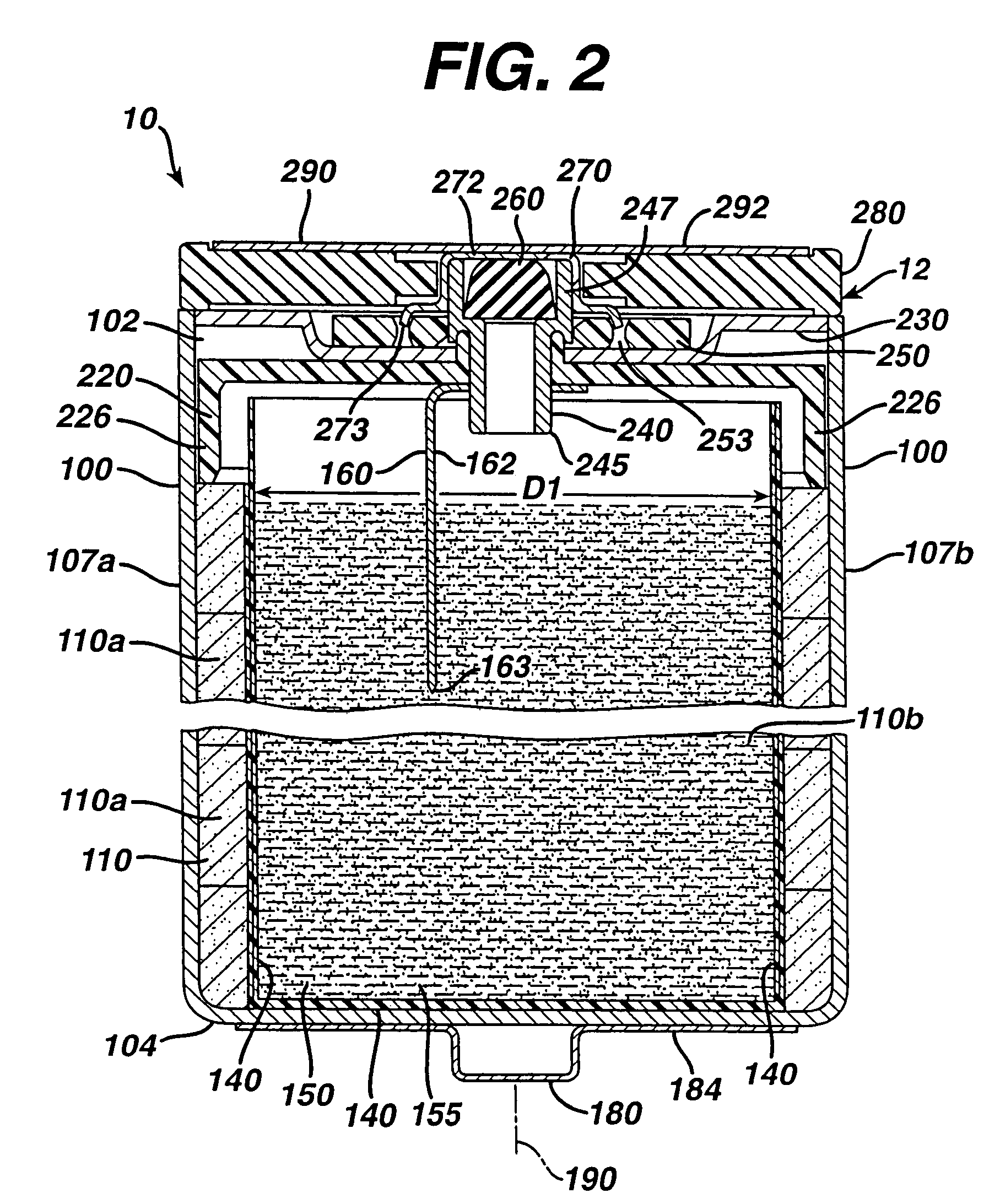
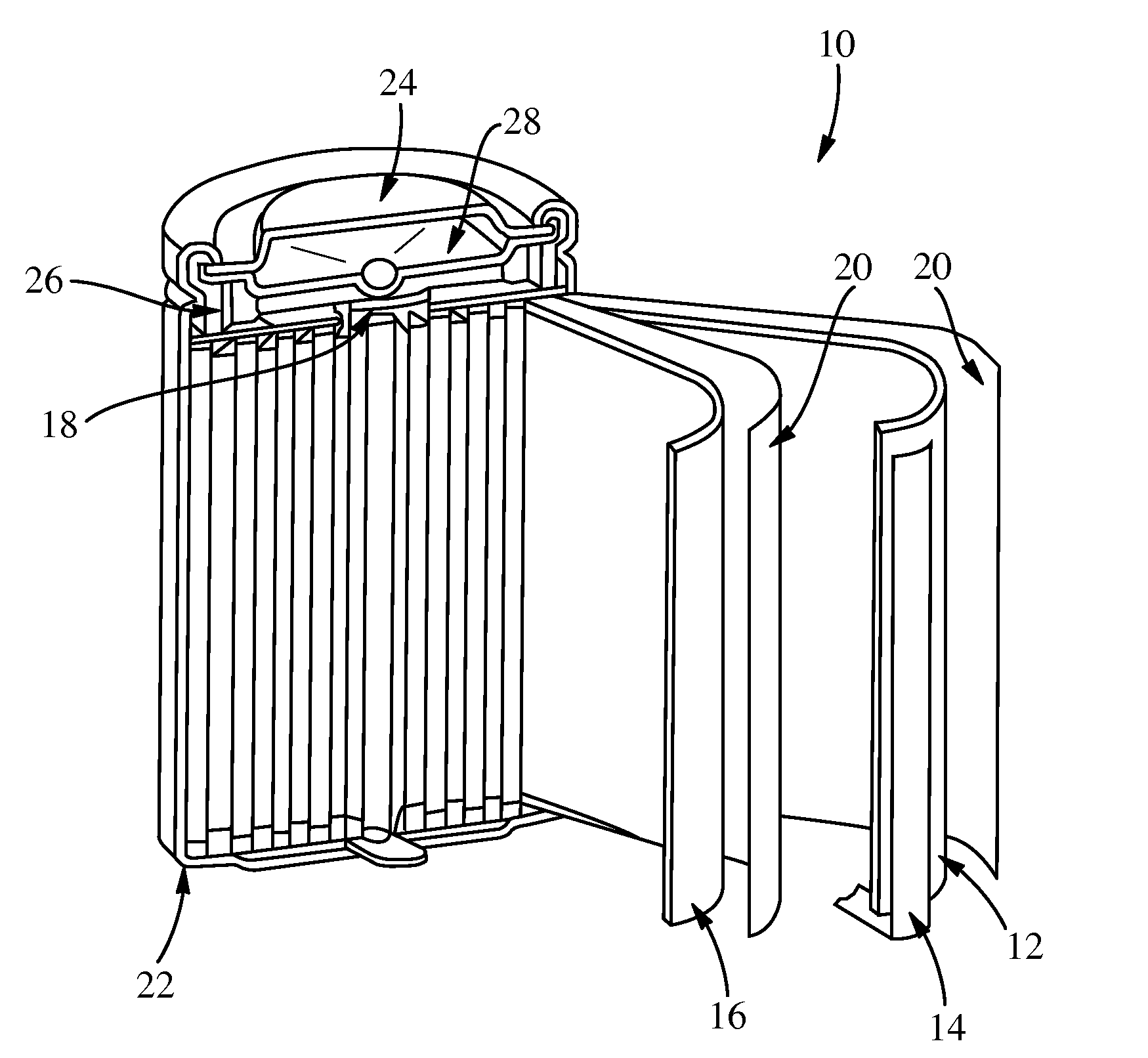
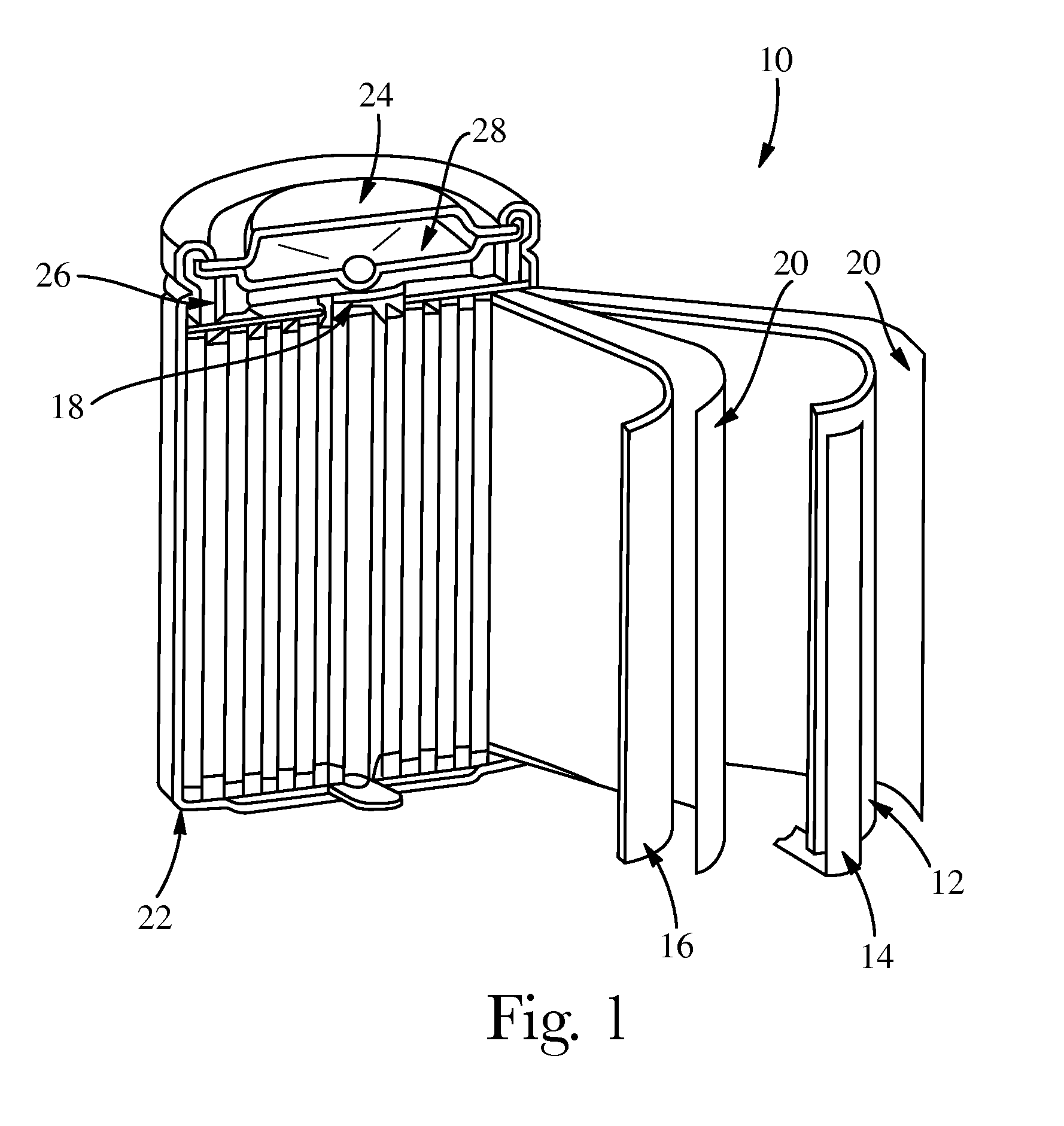
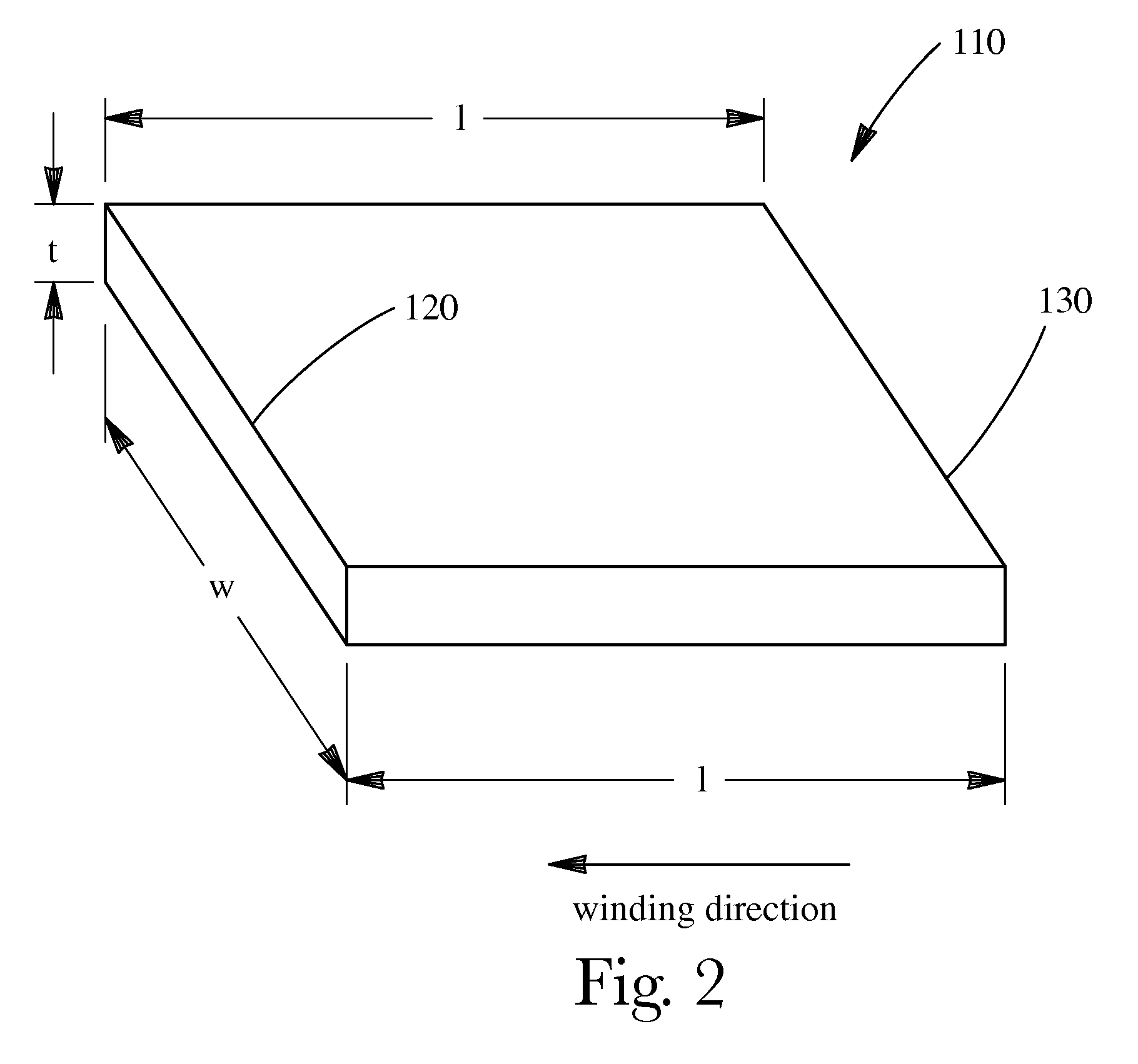
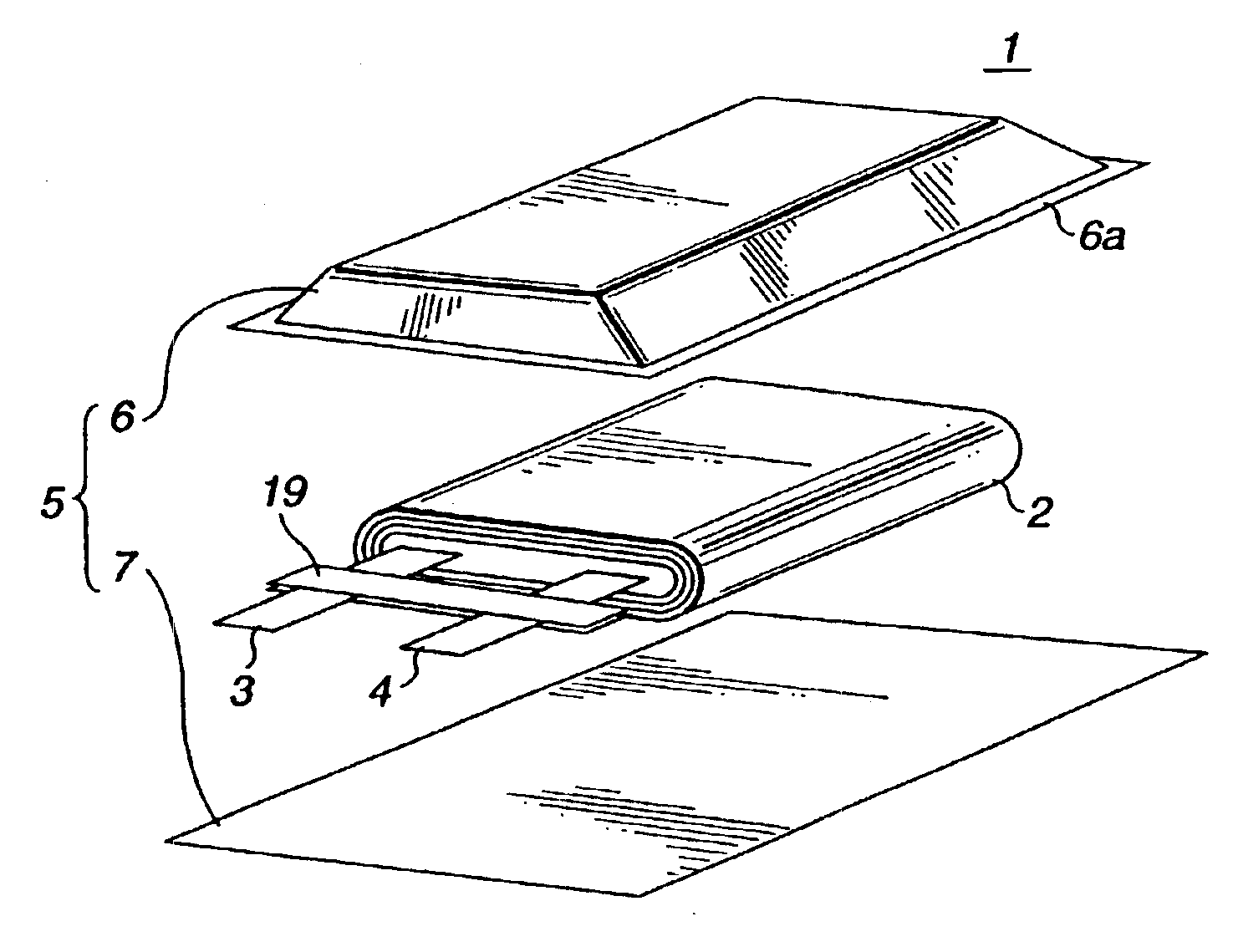
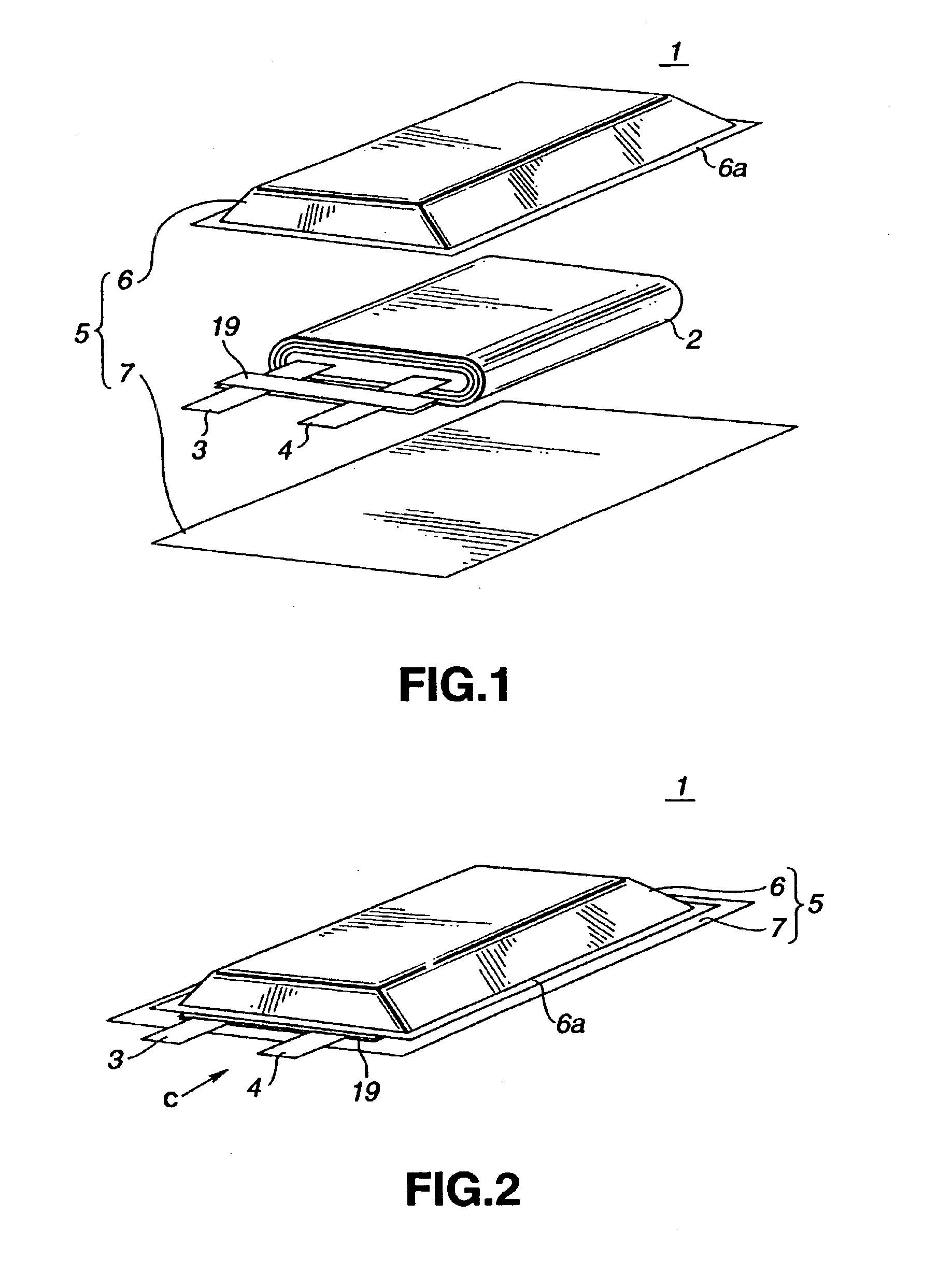
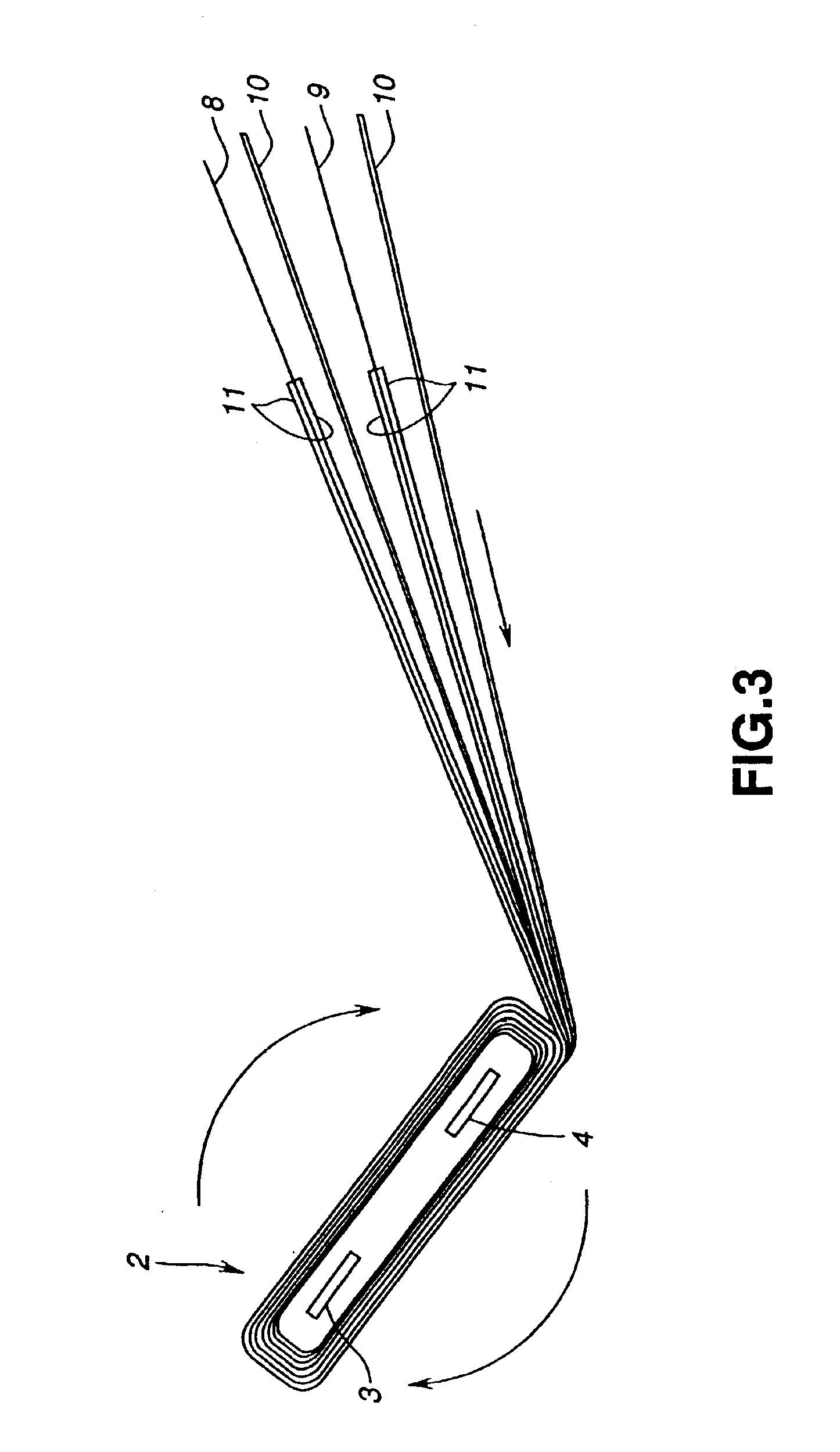
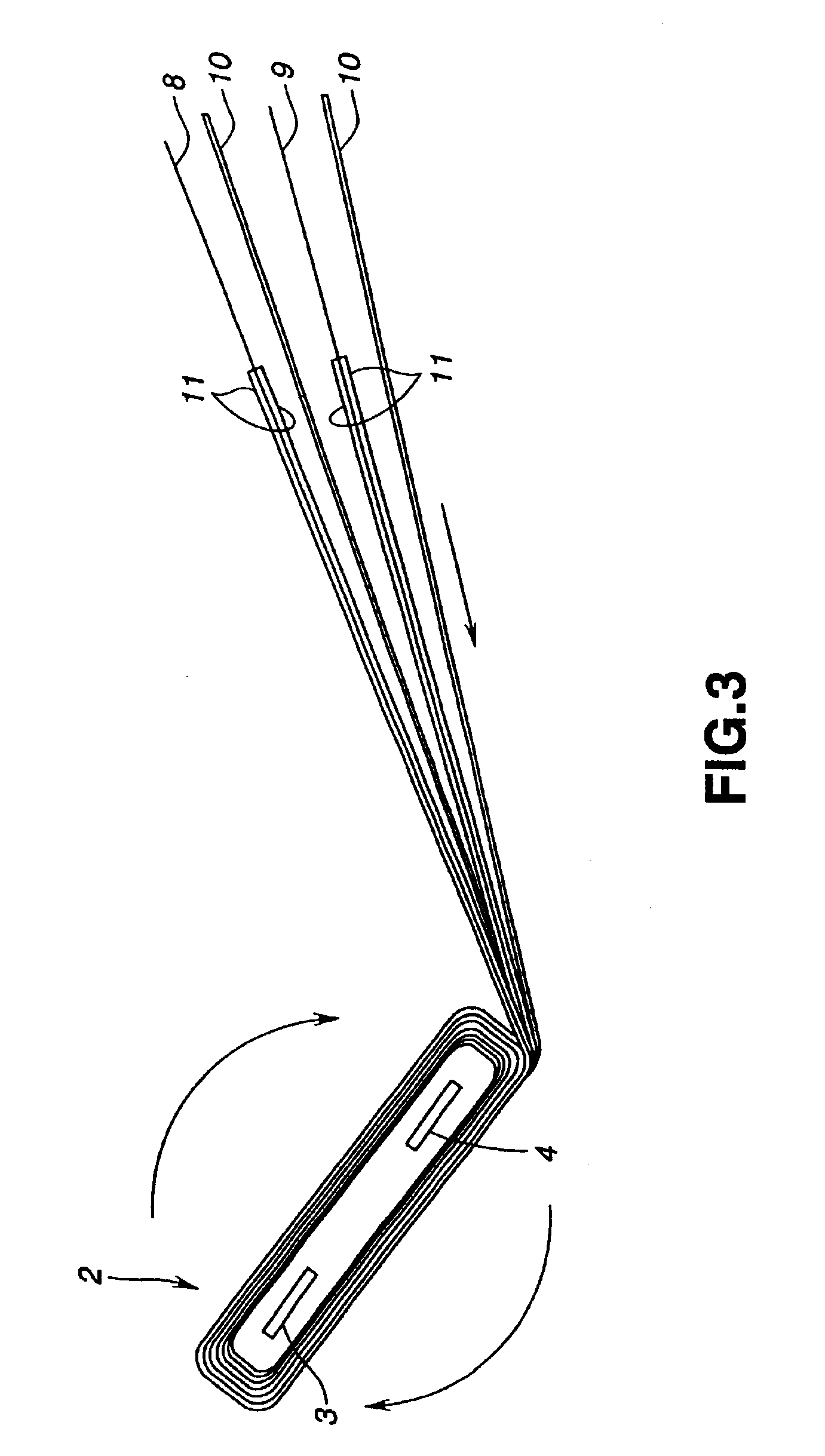
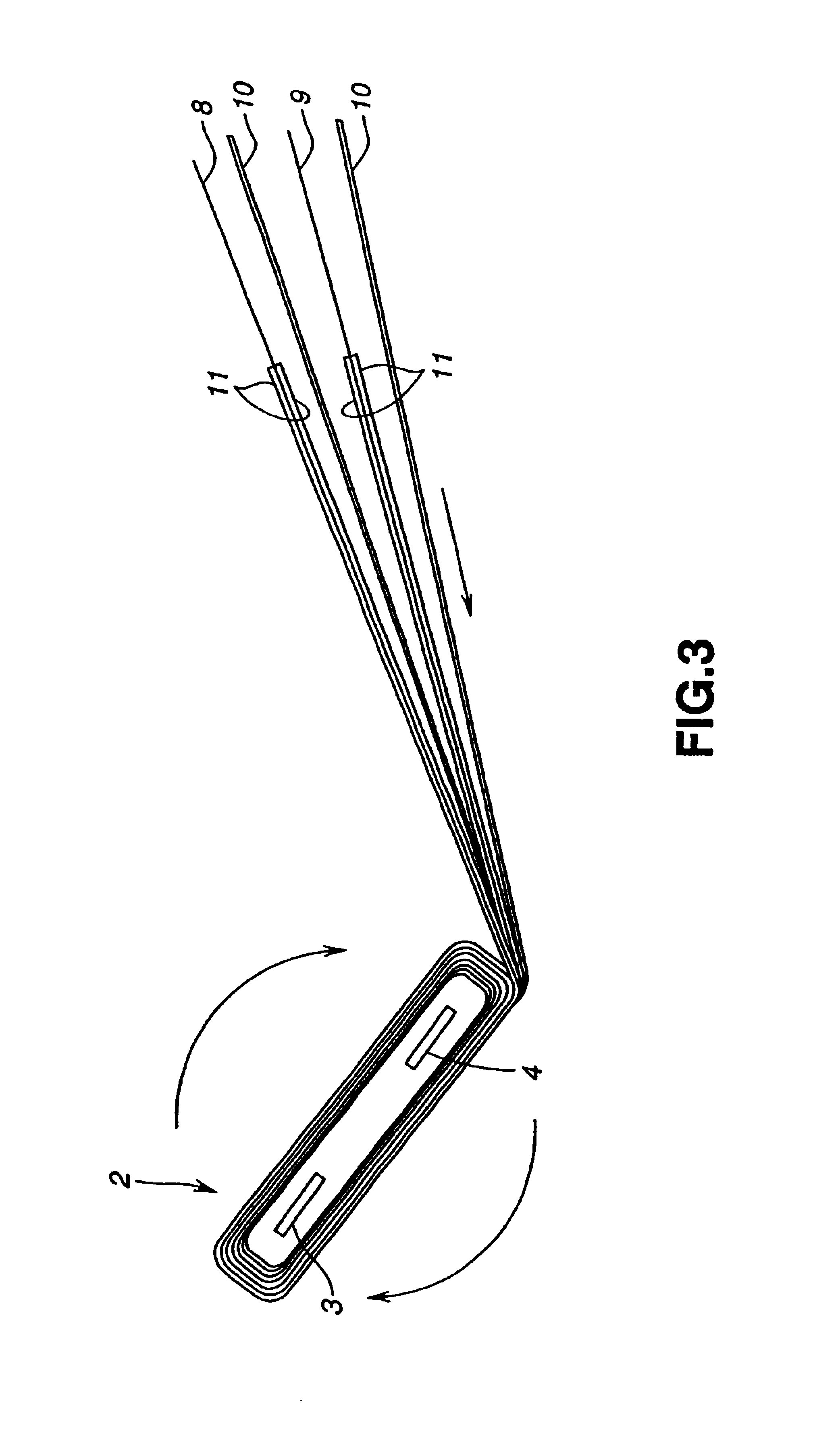
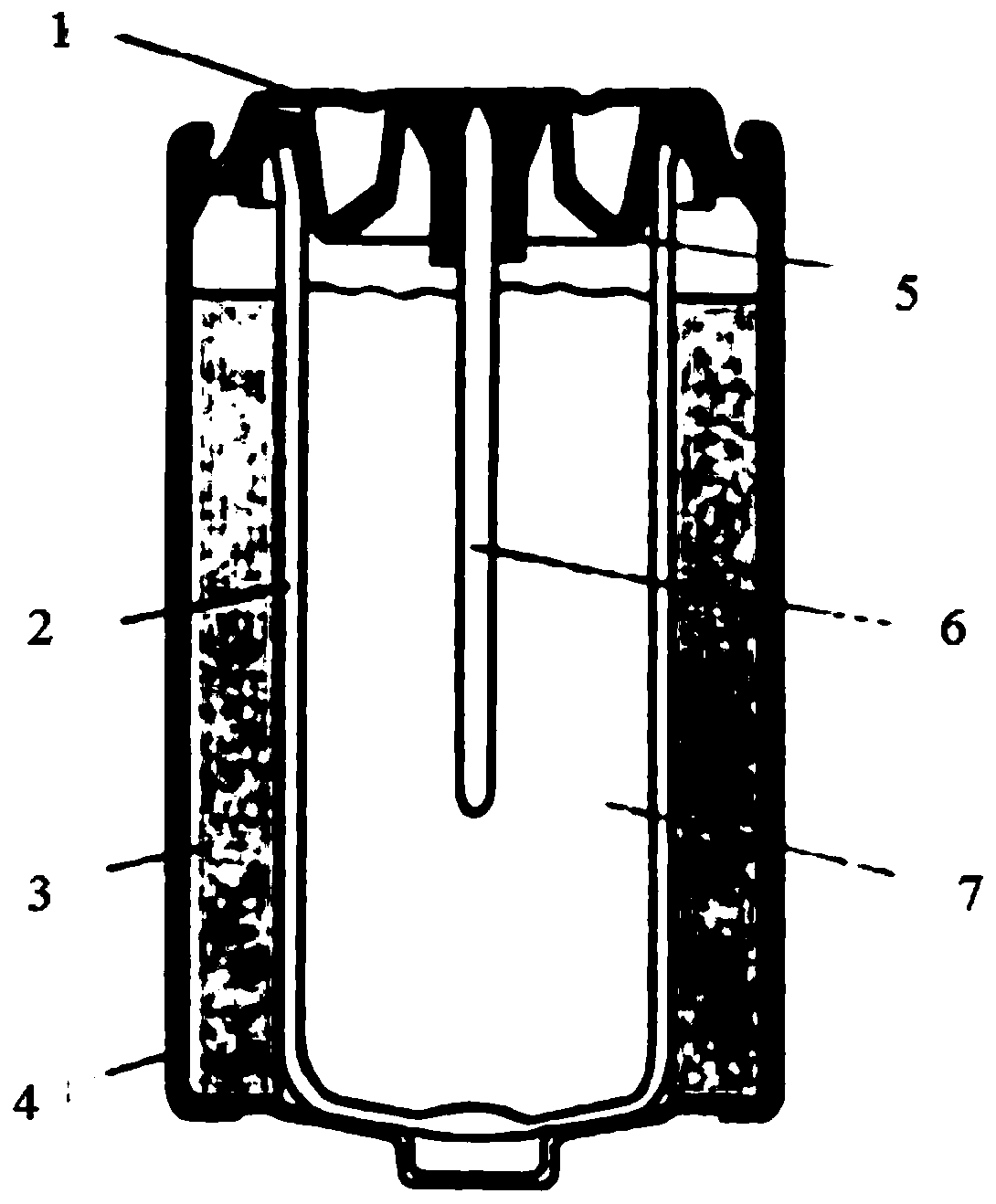
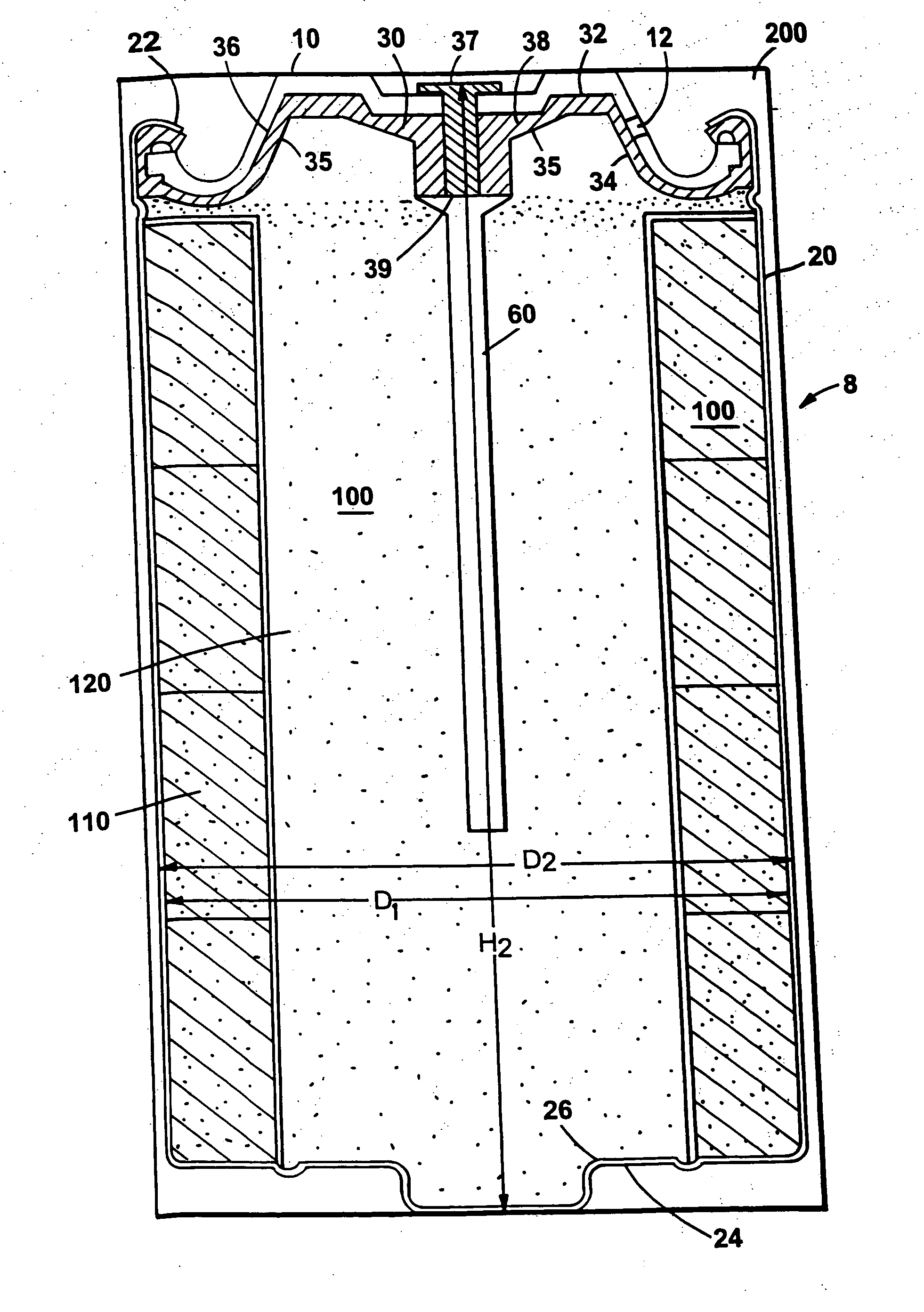
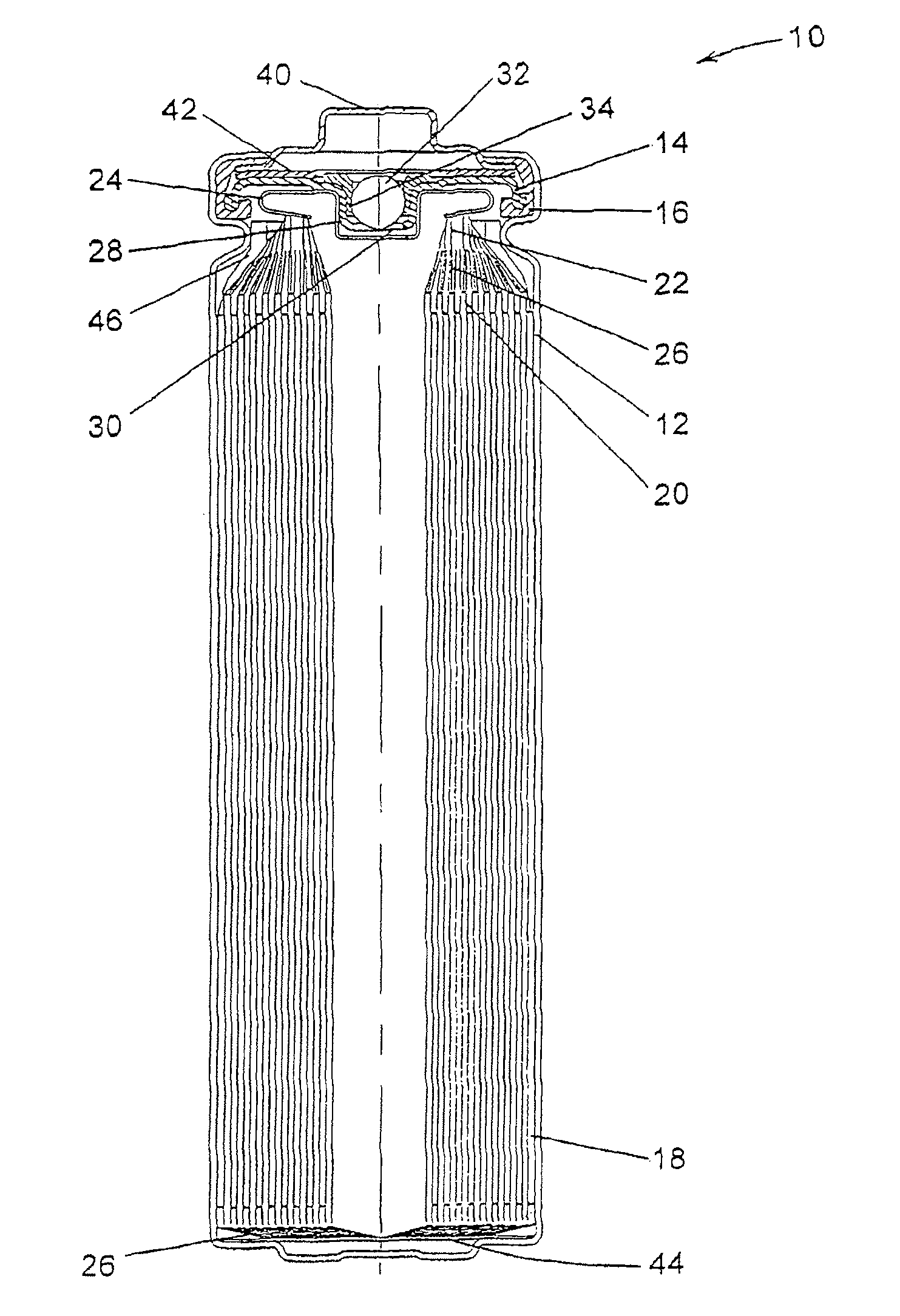
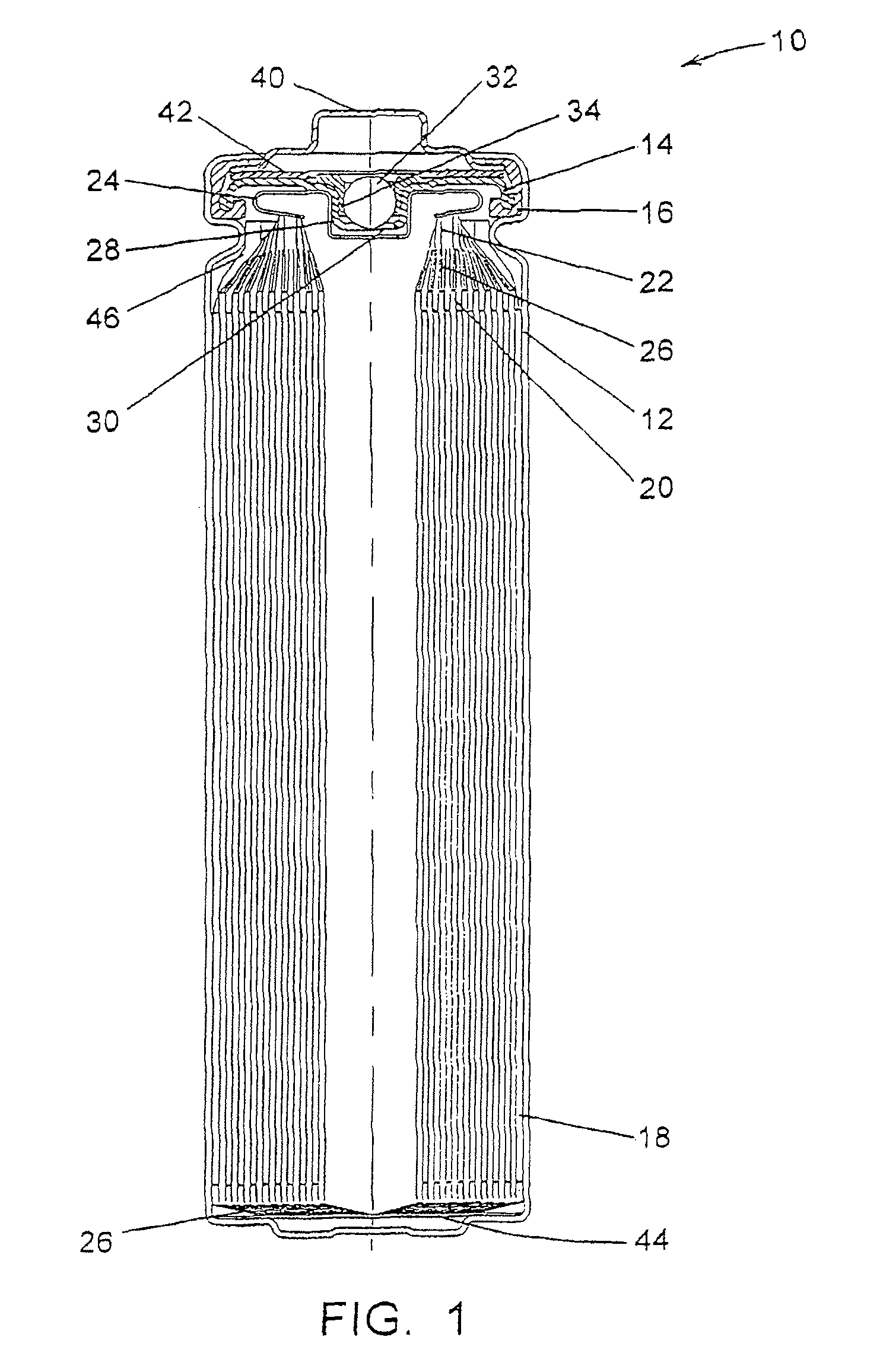
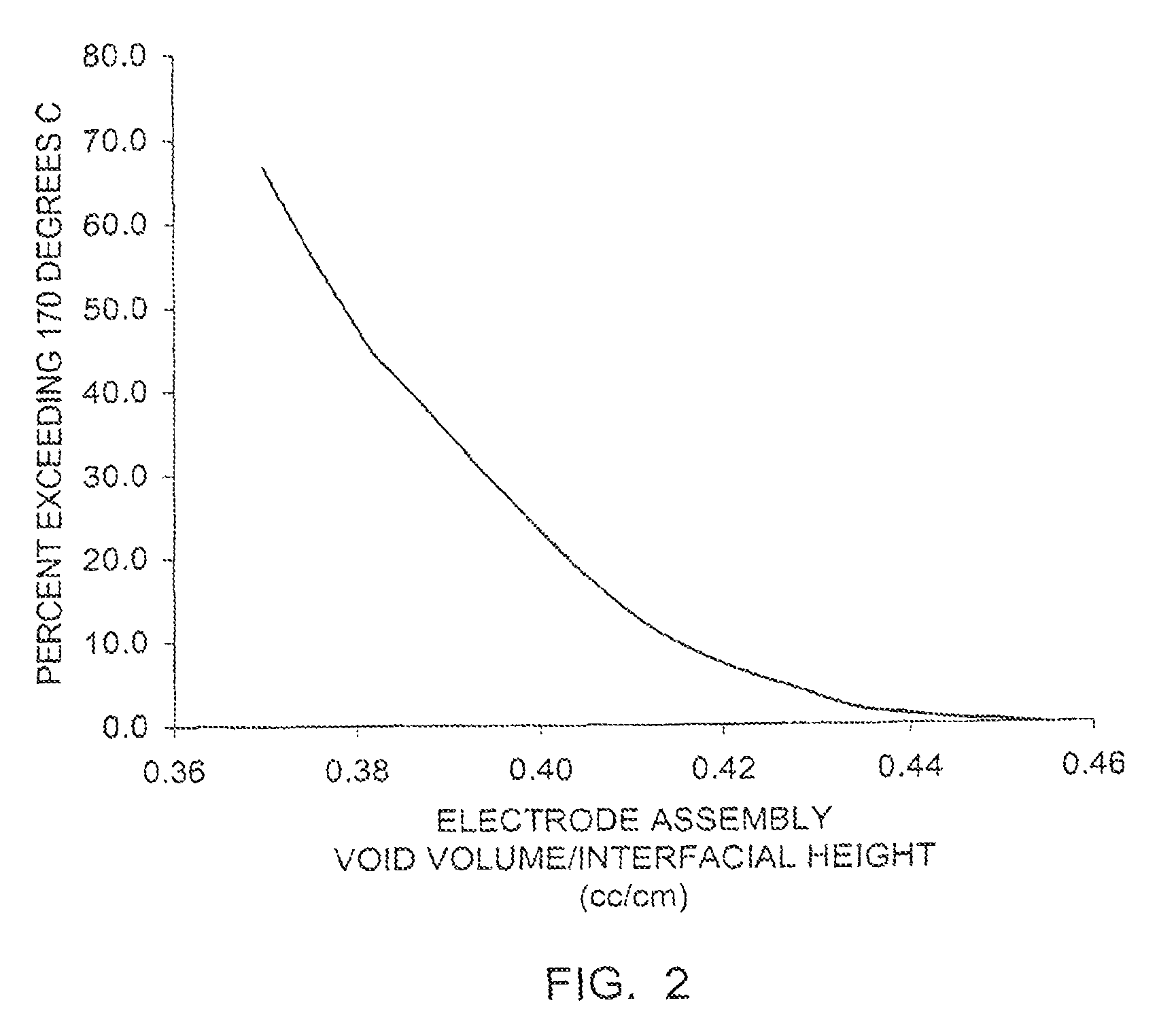
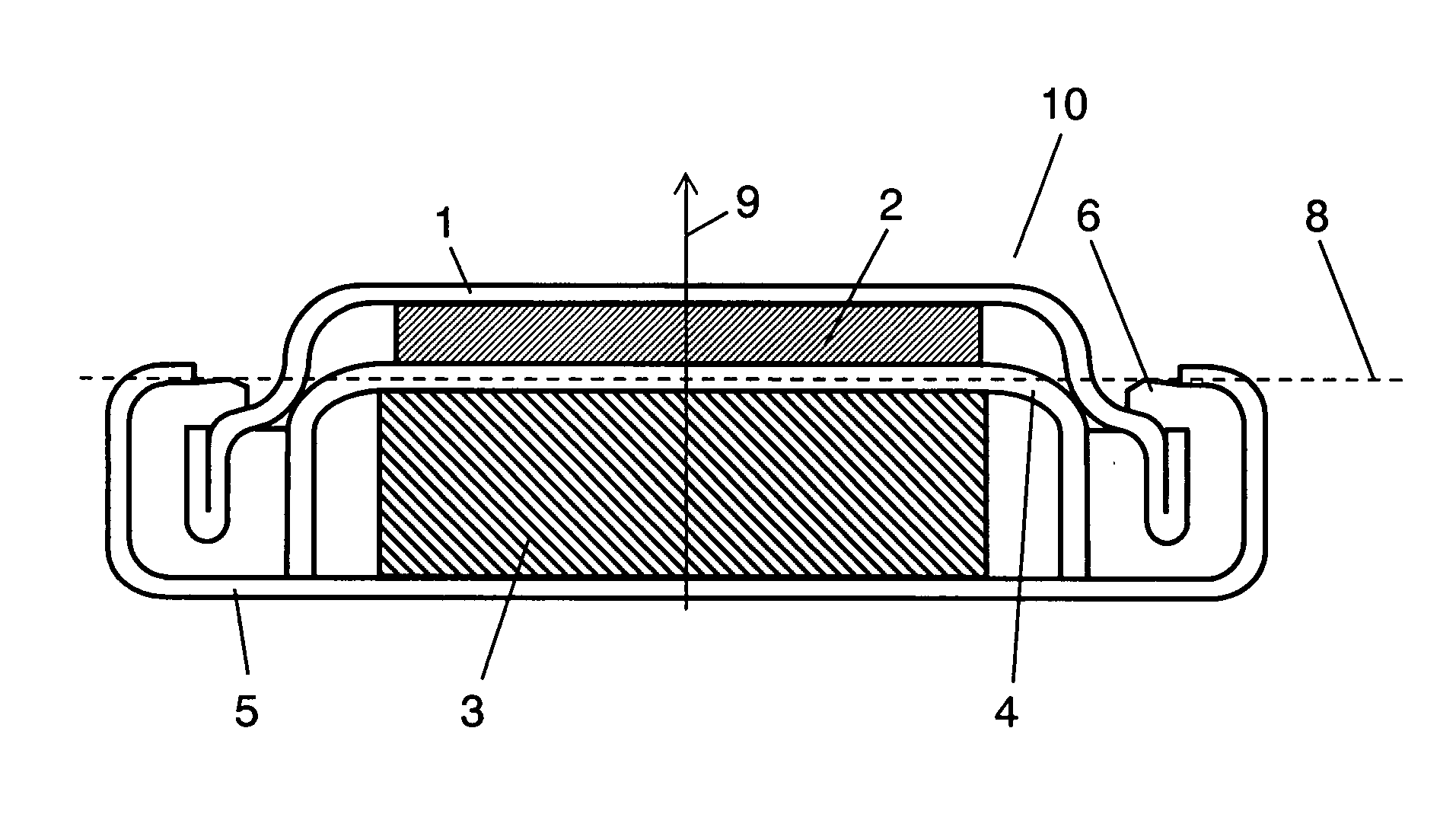
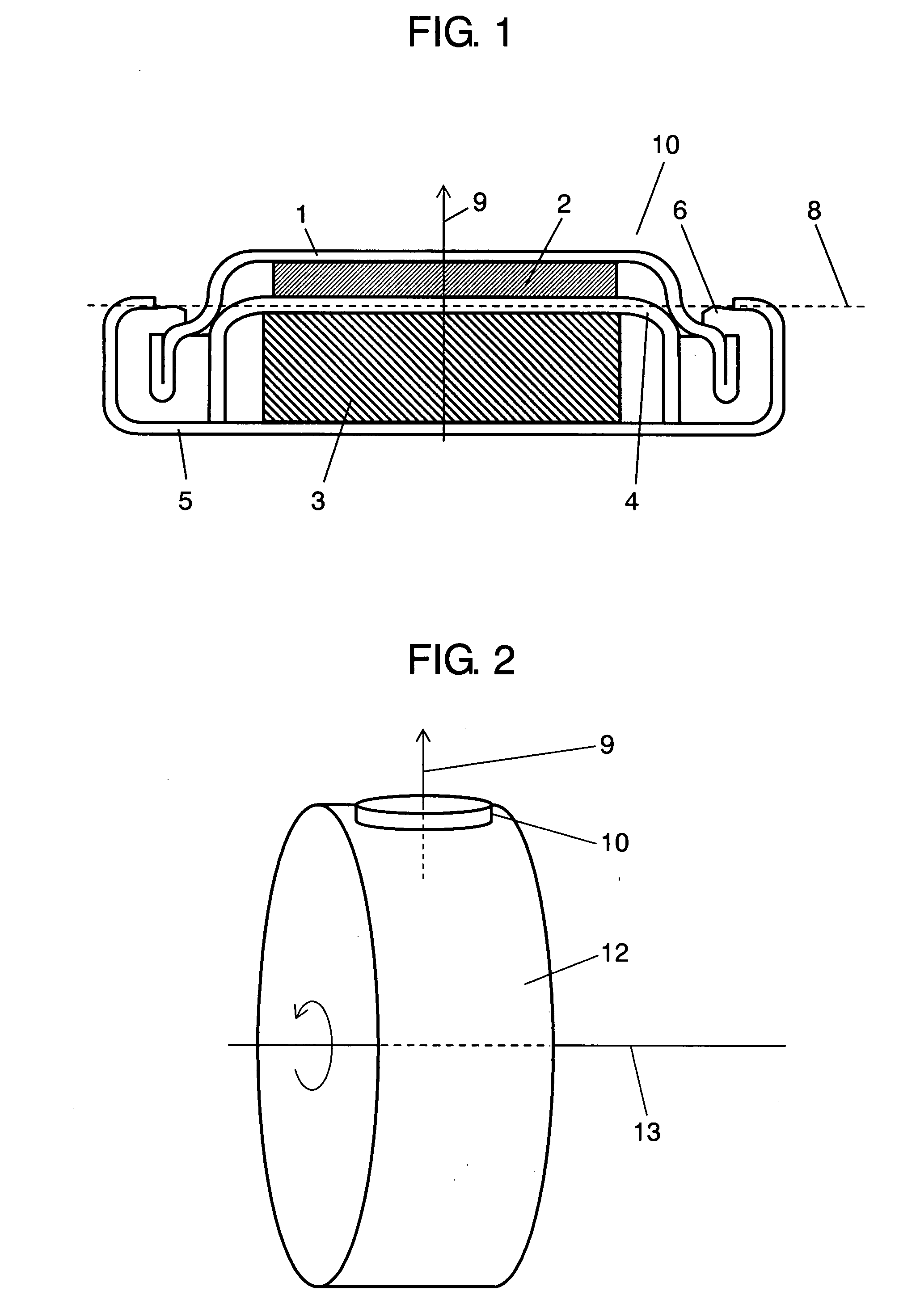
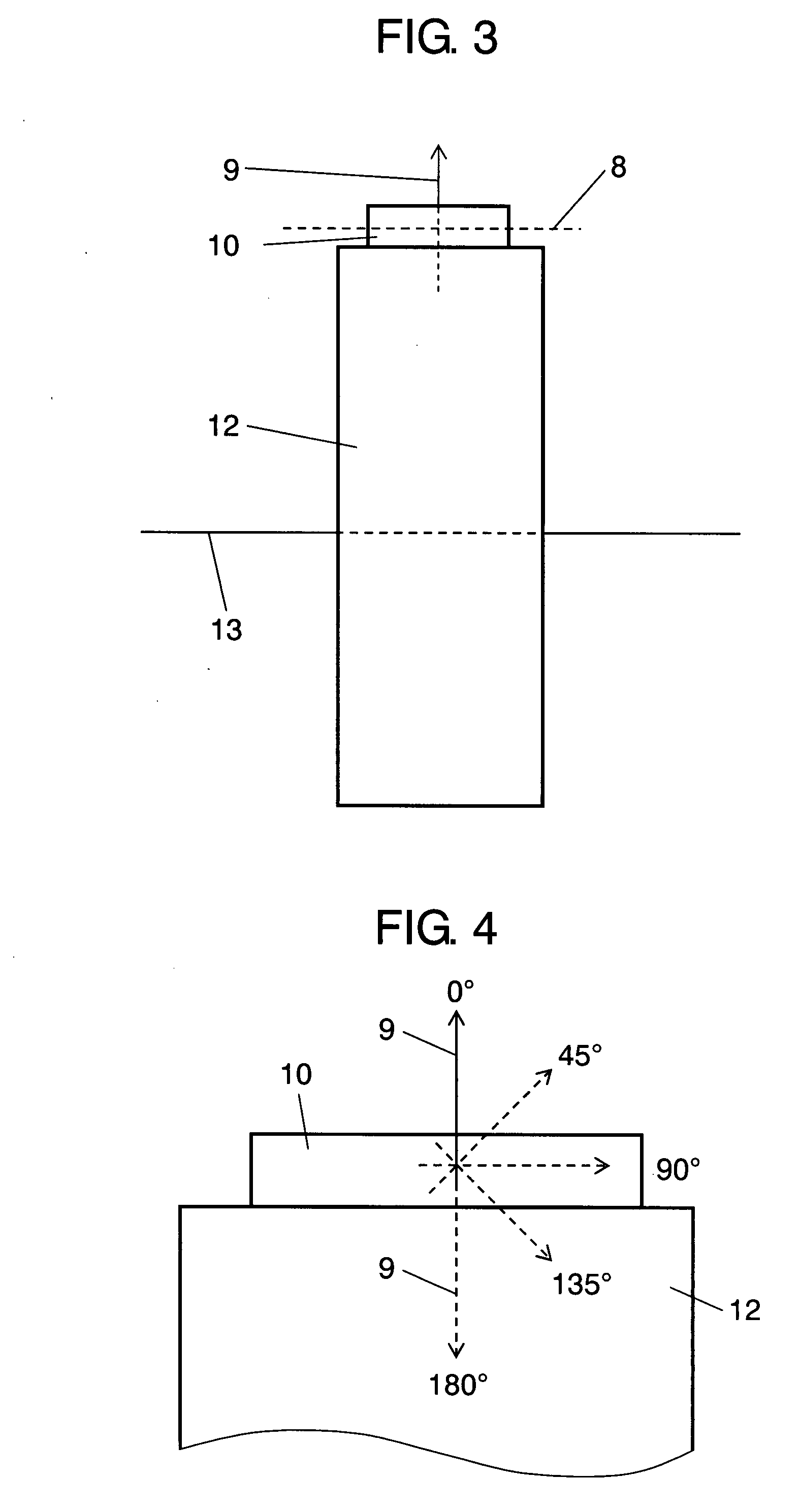
Electrochemical cells with improved spiral-wound electrode assembly
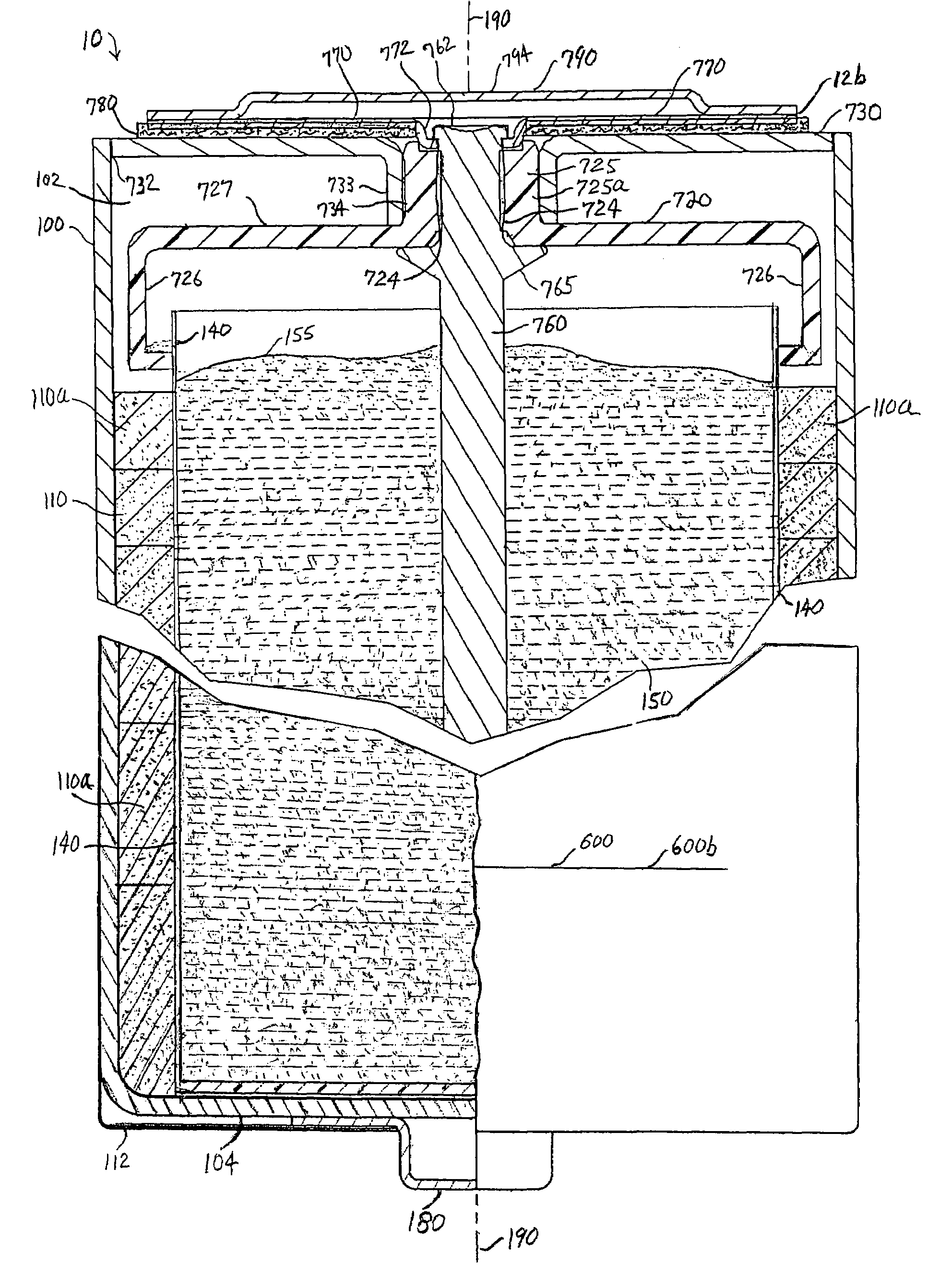
A spiral-wound electrode assembly, an electrochemical cell with the spiral-wound electrode assembly, and a method of manufacturing an electrochemical cell with the spiral-wound electrode assembly are disclosed. The spiral-wound electrode assembly has a first electrode that has a first electrode active material, a leading edge and a trailing edge. The assembly has a second electrode that includes a second electrode active material, a leading edge and a trailing edge. A separator is located between the first electrode and the second electrode. The electrode assembly has an interfacial anode-to-cathode ratio of less than about 1.2. The first electrode, second electrode, and separator are wound so that the first electrode forms a layer at the outermost radius of the electrode assembly and so that the trailing edge of the second electrode extends past the trailing edge of the first electrode.
Owner:DURACELL U S OPERATIONS
High discharge capacity lithium battery
InactiveUS8124274B2Increase capacityProlong lifeElectrode manufacturing processesFinal product manufactureSulfonateElectrical battery
Owner:ENERGIZER BRANDS
Lithium fluoropolymer and fluoro-organic batteries
The invention provides lithium and lithium ion batteries in which the active material of one of the electrodes includes a substantial quantity of a fluoropolymer or fluoro-oligomer material having carbon-fluorine bonds. The fluoropolymer or fluoro-oligomer active material may be mixed with a substantial quantity of electrically conductive material, and may also be mixed with subfluorinated carbonaceous materials. The batteries of the invention are useful for elevated temperature applications. The invention also provides methods for electrochemical generation of energy which employ the batteries of the invention at elevated temperatures.
Owner:CALIFORNIA INST OF TECH
Noble metals coated on titanium current collectors for use in nonaqueous Li/CFx cells
A lithium / fluorinated carbon electrochemical cell having the CFx material supported on a titanium current collector screen sputter coated with a noble metal is described. The gold, iridium, palladium, platinum, rhodium and ruthenium-coated titanium current collector provides the cell with higher rate capability, even after exposure to high temperatures, in comparison to cells of a similar chemistry having the CFx contacted to a titanium current collector painted with a carbon coating.
Owner:WILSON GREATBATCH LTD
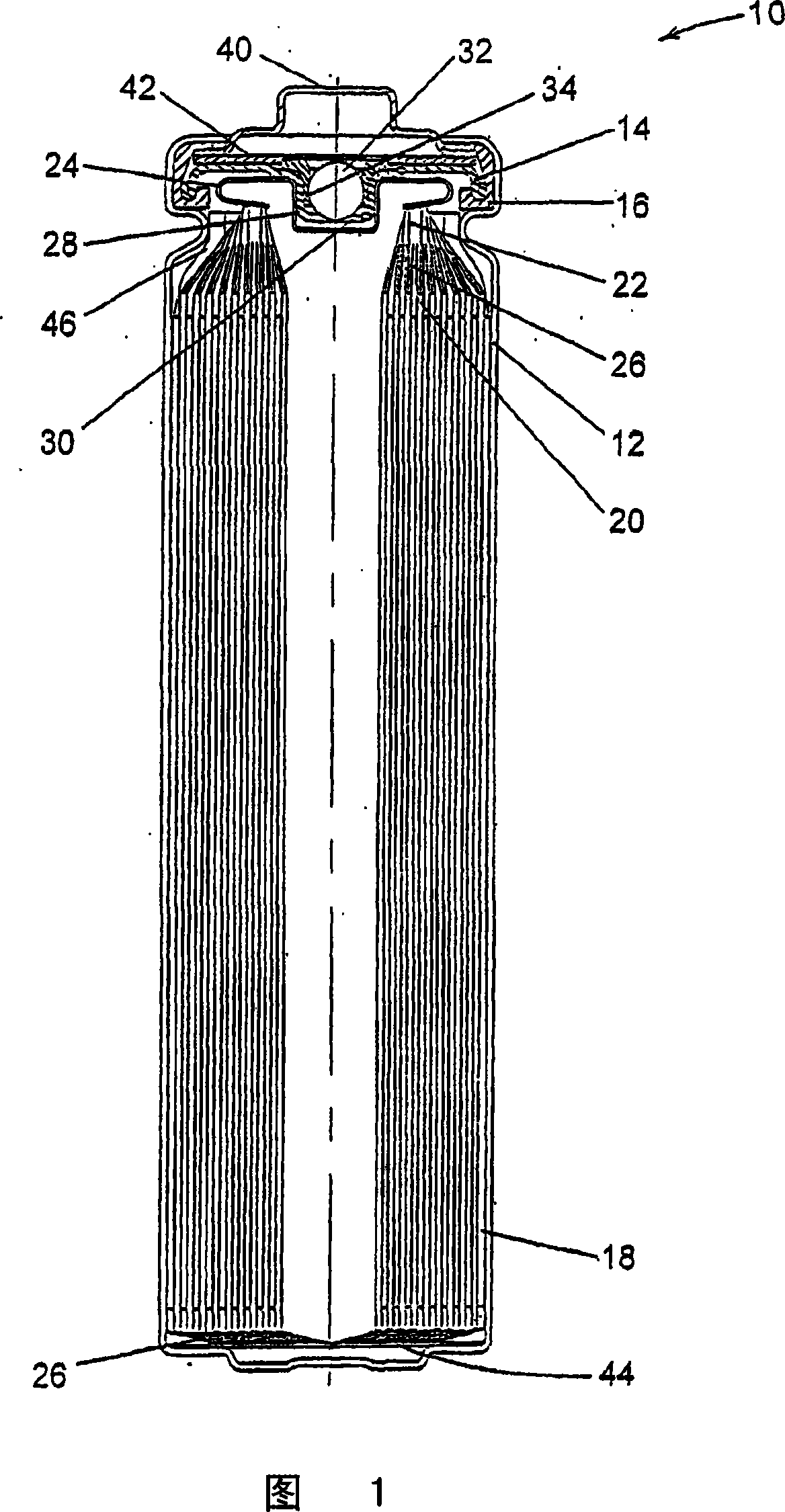
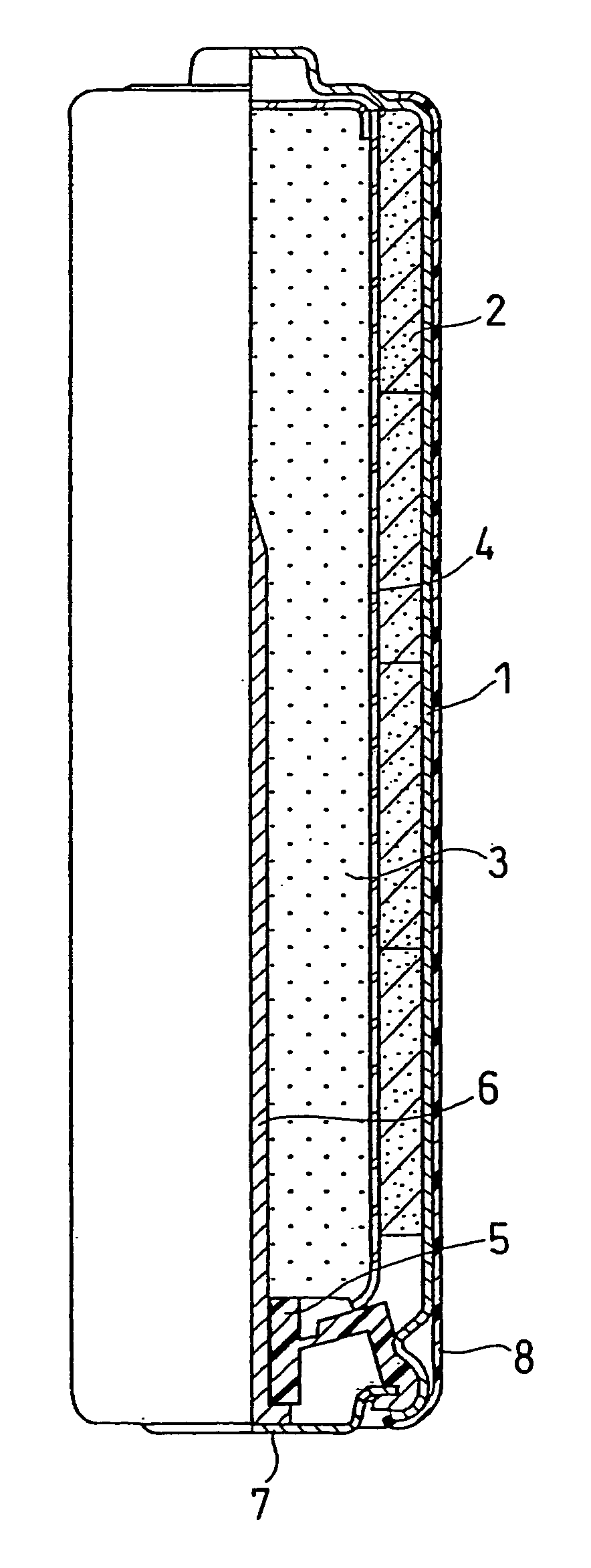
Solid electrolyte battery
InactiveUS6921607B2Increase energy densityFinal product manufactureSolid electrolyte cellsTotal thicknessPolymer chemistry
A solid electrolyte battery is disclosed incorporating a wound electrode having a positive electrode with a collector having two sides on which positive-electrode active material layers are formed, a negative electrode with a collector having two sides on which negative-electrode active material layers are formed, and a solid electrolyte layer formed between the positive electrode and the negative electrode a total film thickness A of the positive-electrode active material layers satisfies a range from 60 mm to 150 mm, and a ratio A / B of the total film thickness A of the positive-electrode active material layers with respect to a total thickness B of the negative-electrode active material layers satisfies a range from 0.5 to 1.2.
Owner:SONY CORP
Solid electrolyte battery
InactiveUS6933073B2Increase energy densityFinal product manufactureSolid electrolyte cellsElectrical batteryBattery cell
A solid electrolyte battery is disclosed incorporating a wound electrode having a positive electrode with a collector having two sides on which positive-electrode active material layers are formed, a negative electrode with a collector having two sides on which negative-electrode active material layers are formed, and a solid electrolyte layer formed between the positive electrode and the negative electrode, wherein a total film thickness A of the positive-electrode active material layers satisfies a range from 60 mm to 150 mm, and a ratio A / B of the total film thickness A of the positive-electrode active material layers with respect to a total thickness B of the negative-electrode active material layers satisfies a range from 0.5 to 1.2.
Owner:SONY CORP
Solid electrolyte battery
InactiveUS6926993B2Increase energy densityFinal product manufactureSolid electrolyte cellsTotal thicknessPolymer chemistry
A solid electrolyte battery is disclosed incorporating a wound electrode having a positive electrode with a collector having two sides on which positive-electrode active material layers are formed, a negative electrode with a collector having two sides on which negative-electrode active material layers are formed, and a solid electrolyte layer formed between the positive electrode and the negative electrode, wherein a total film thickness A of the positive-electrode active material layers satisfies a range from 60 mm to 150 mm, and a ratio A / B of the total film thickness A of the positive-electrode active material layers with respect to a total thickness B of the negative-electrode active material layers satisfies a range from 0.5 to 1.2.
Owner:SONY CORP
Alkaline battery
InactiveUS7553586B2Excellent characteristicsIncrease resistanceFuel and primary cellsPrimary cell electrodesElectrolyte leakageHigh rate
An alkaline battery includes: a negative electrode including a zinc or zinc alloy powder as an active material; an alkaline electrolyte; and a positive electrode. The zinc or zinc alloy powder has a specific surface area of 0.01 to 10 m2 / g, and the weight ratio of the electrolyte to the negative electrode active material is in the range of 0.1 to 2. This invention can provide an alkaline battery that is improved in electrolyte leakage resistance and high-rate discharge characteristics.
Owner:PANASONIC CORP
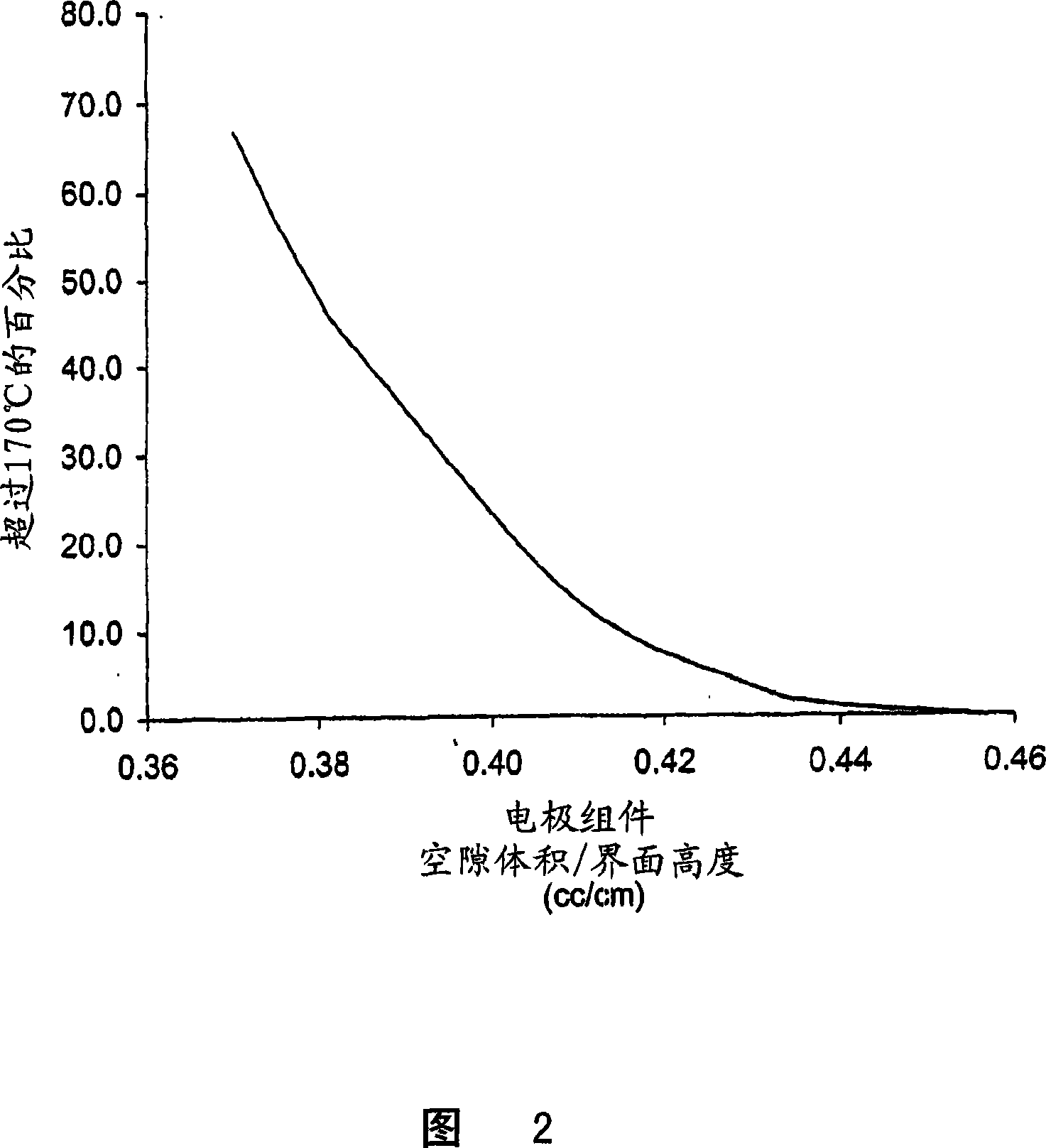
Semi-solid battery positive electrode material and alkaline zinc-manganese battery prepared from same
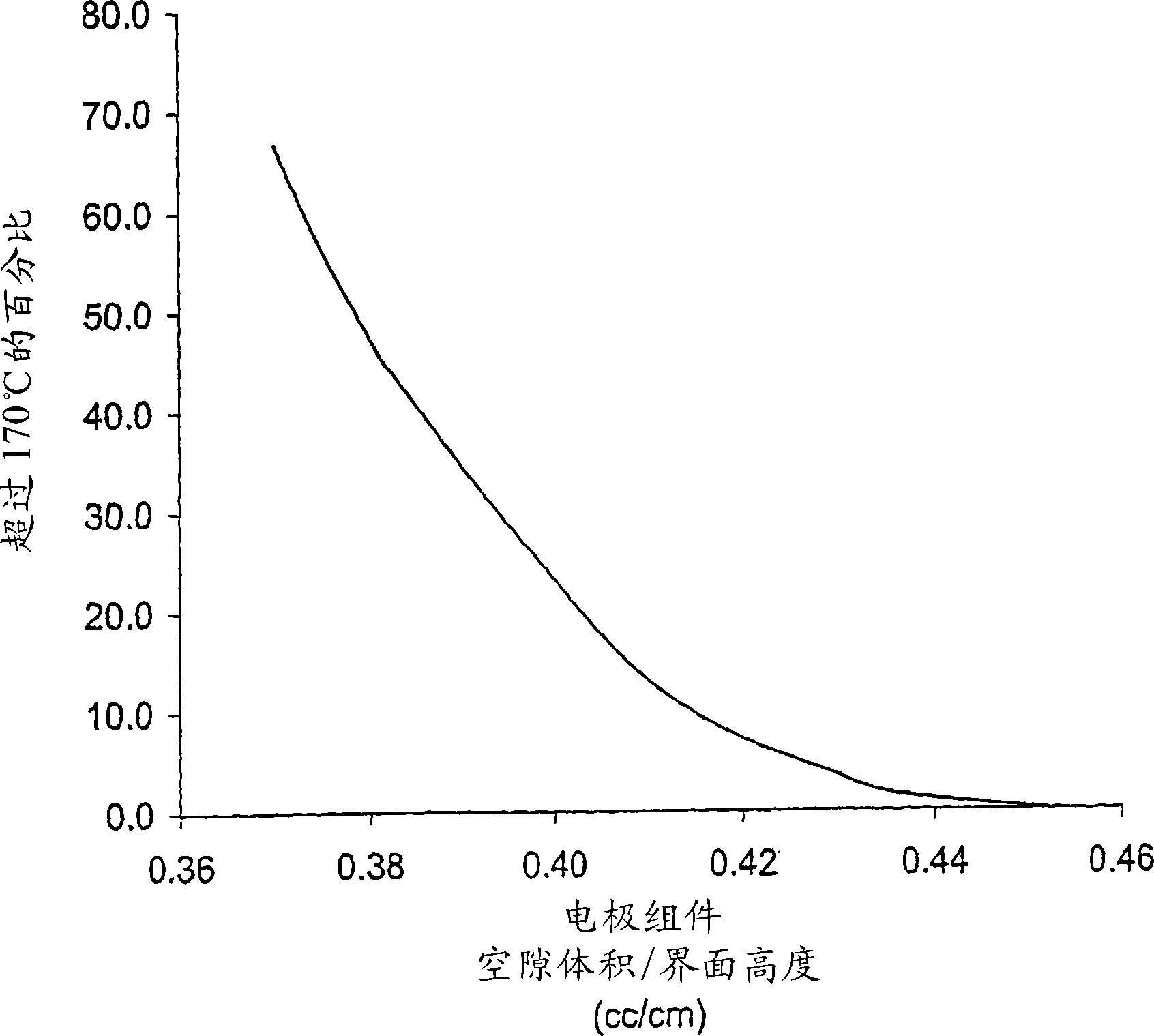
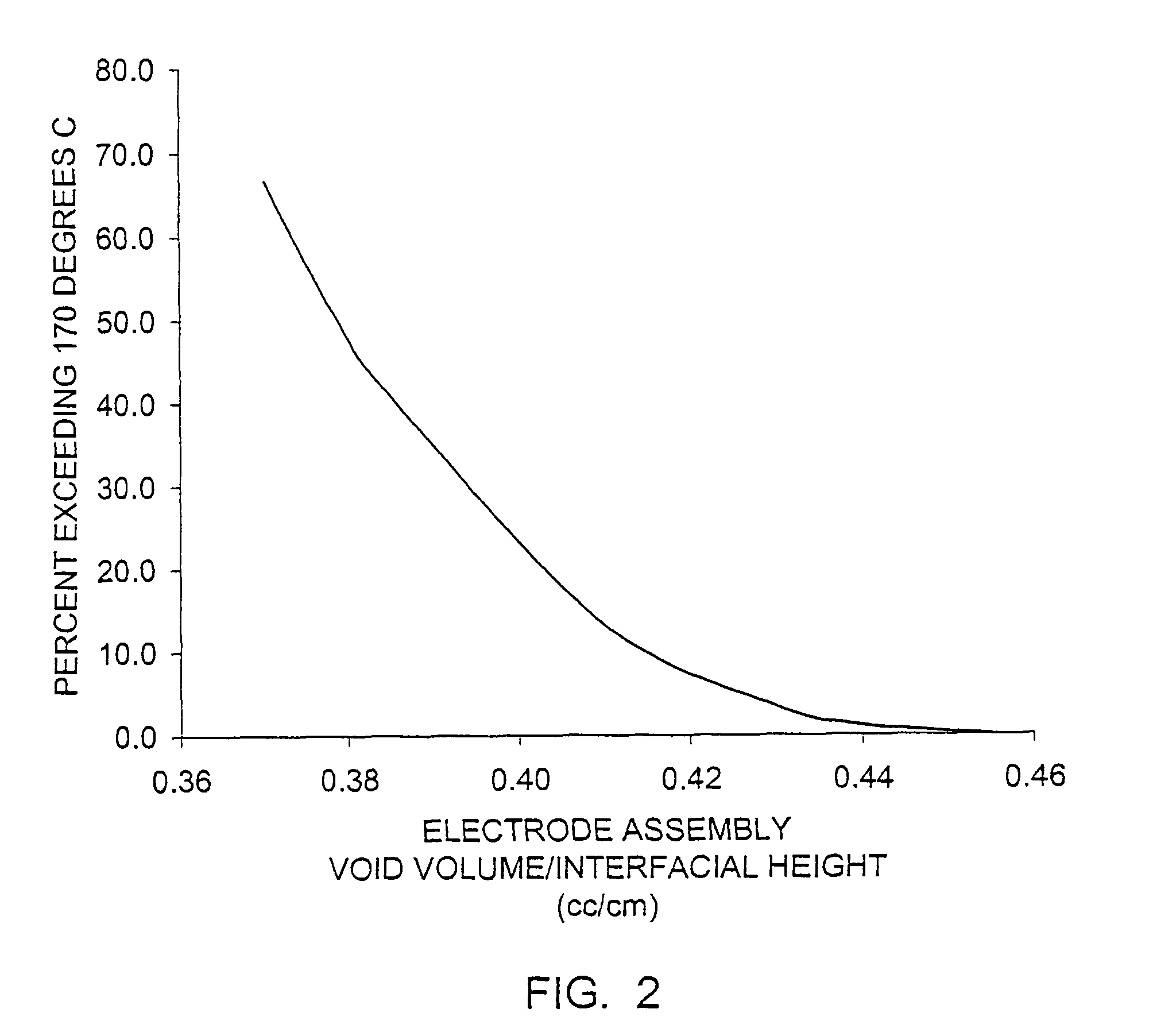
InactiveCN110739459AReduce polarizationImprove discharge performanceElectrode manufacturing processesElectrolyte/electrodes capacity ratioElectrolytic agentElectrical battery
The invention belongs to the technical field of chemical batteries and particularly relates to a semi-solid battery positive electrode material and an alkaline zinc-manganese battery prepared from thesame. The semi-solid battery positive electrode material is a semi-solid material with a liquid-solid weight ratio of about 0.47-0.52. The positive electrode material is prepared from the following components of: 170 to 175 parts of electrolytic manganese dioxide (EMD); 13 to 15 parts of graphite; 90 to 95 parts of electrolyte; 0.18 to 0.22 part of hydrated titanium dioxide; and 0.16 to 0.20 partof zinc stearate. With the semi-solid battery positive electrode material adopted, the internal polarization phenomenon of a battery during high-current discharge can be greatly reduced; conductivityis optimized; the discharge performance of an alkaline manganese battery during large-load and medium-load discharge is improved; the discharge duration of the alkaline manganese battery during large-load and medium-load discharge is prolonged; the void ratio of a positive electrode ring and the content of electrolyte are increased; sufficient reaction can be achieved during large-current discharge; and the utilization rate of an active substance manganese dioxide is increased.
Owner:NINGBO BEITERUI ENERGY TECH
Alkaline cell
InactiveCN101304086AAvoid polarizationCell preserving/storagePrimary cell electrodesSimple Organic CompoundsDepolarizer
An alkaline dry battery includes a positive electrode, a negative electrode, a separator, and an alkaline electrolyte. The separator is provided between the positive electrode and the negative electrode, and the positive electrode, the negative electrode, and the separator are impregnated with the alkaline electrolyte. A battery depolarizer, which is an organic compound having a function of depolarizing both the positive electrode and the negative electrode or an alkaline metal salt thereof, is added to at least the alkaline electrolyte.
Owner:PANASONIC CORP
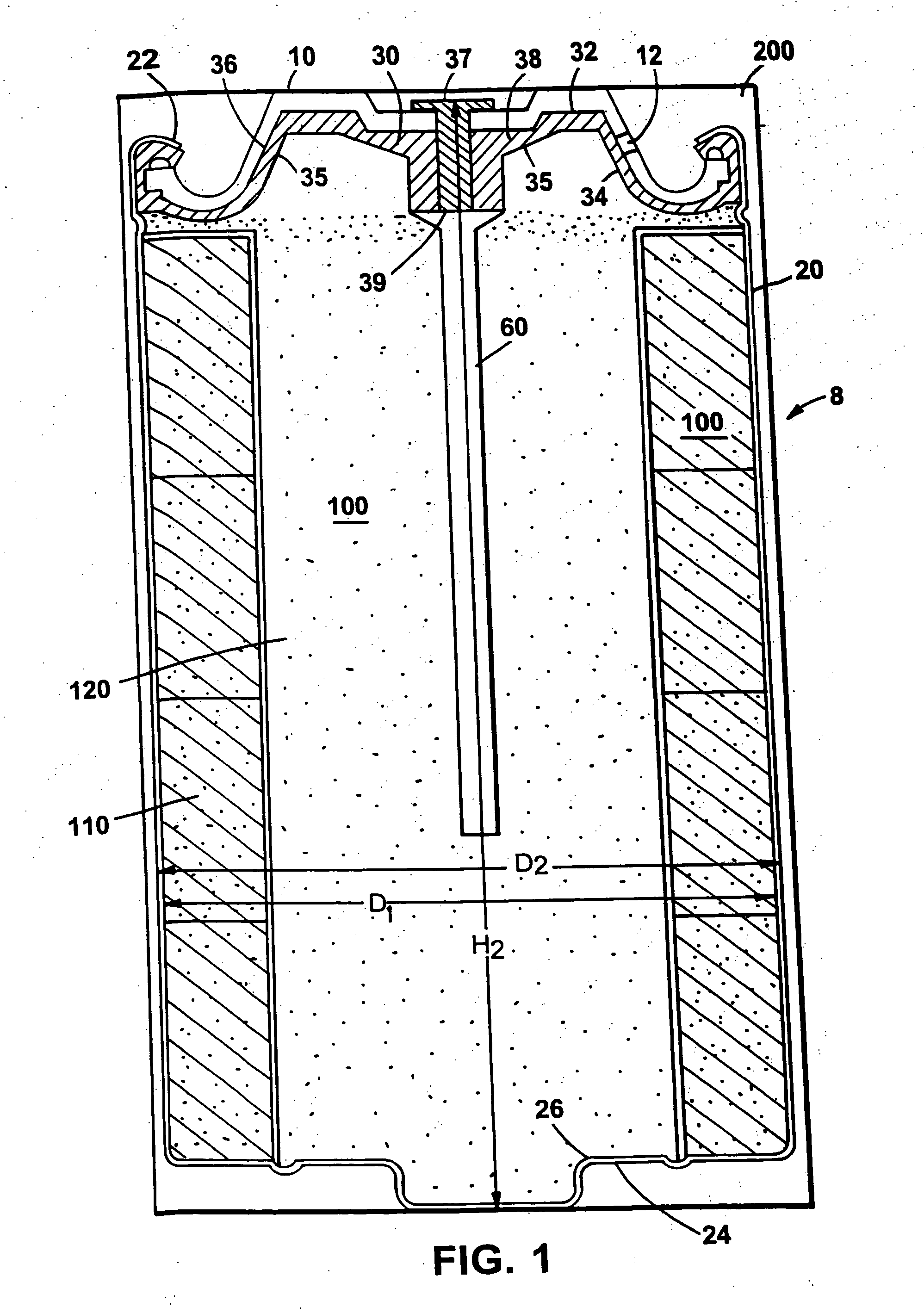
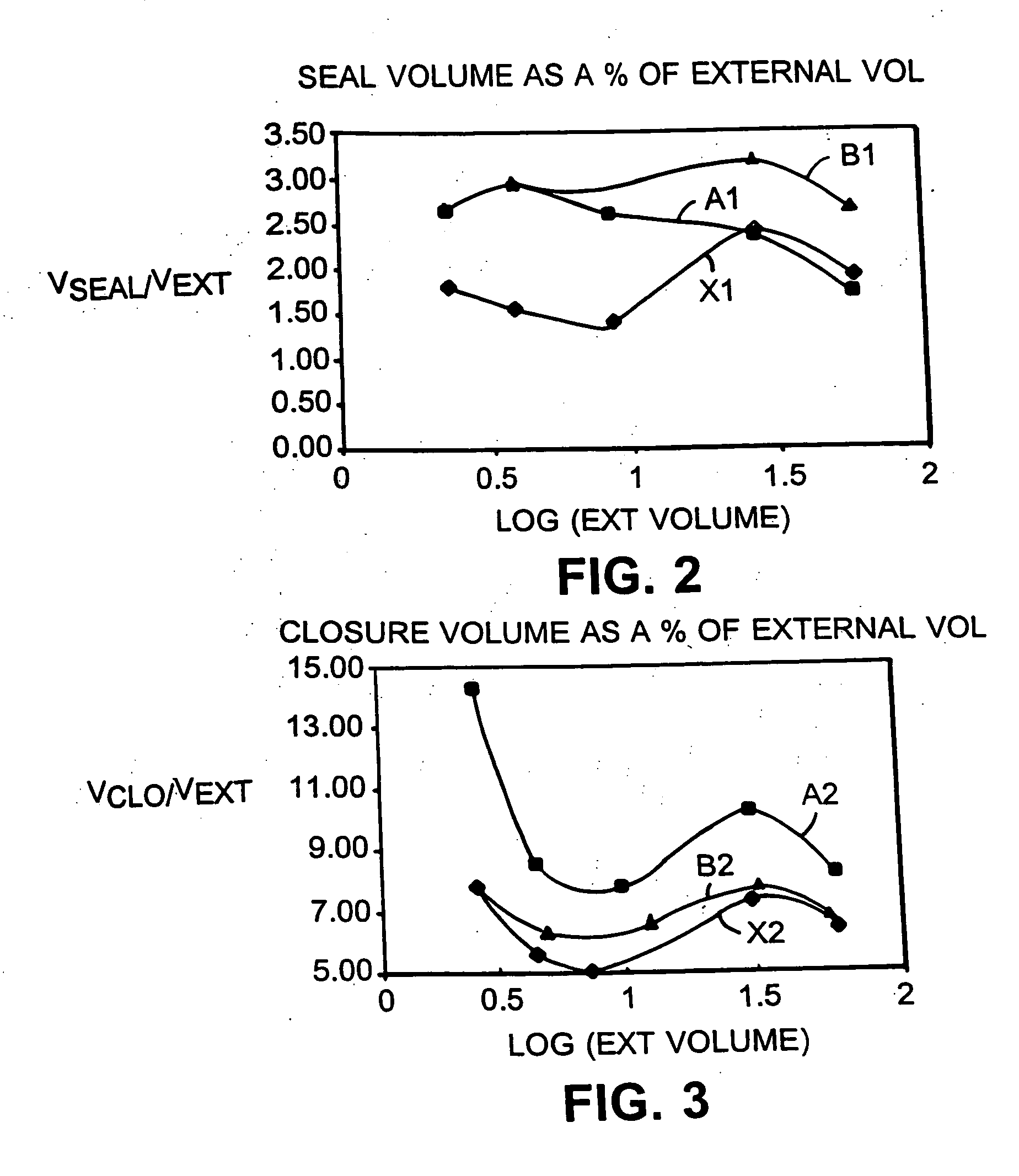
Electrochemical cell closure
InactiveUS20050147879A1Large capacityIncrease capacityAlkaline accumulatorsFinal product manufactureEngineeringSpecific volume
An electrochemical cell having a high capacity is described. The cell capacity can be increased by a method of selecting cell components to achieve particular volume ratios within the cell. Specific volume ratios that lead to improved capacity include the ratio of the internal cell volume to the external volume, the ratio of the closure volume to the external volume, the ratio of the closure volume to the internal cell volume, the ratio of the seal volume to the internal cell volume, and the ratio of the seal volume to the external volume.
Owner:THE GILLETTE CO
High discharge capacity lithium battery
InactiveUS8007940B2Improve discharge performanceIncrease energy densityOrganic electrolyte cellsActive material electrodesHigh rateIron disulfide
Owner:ENERGIZER BRANDS
Flat Battery
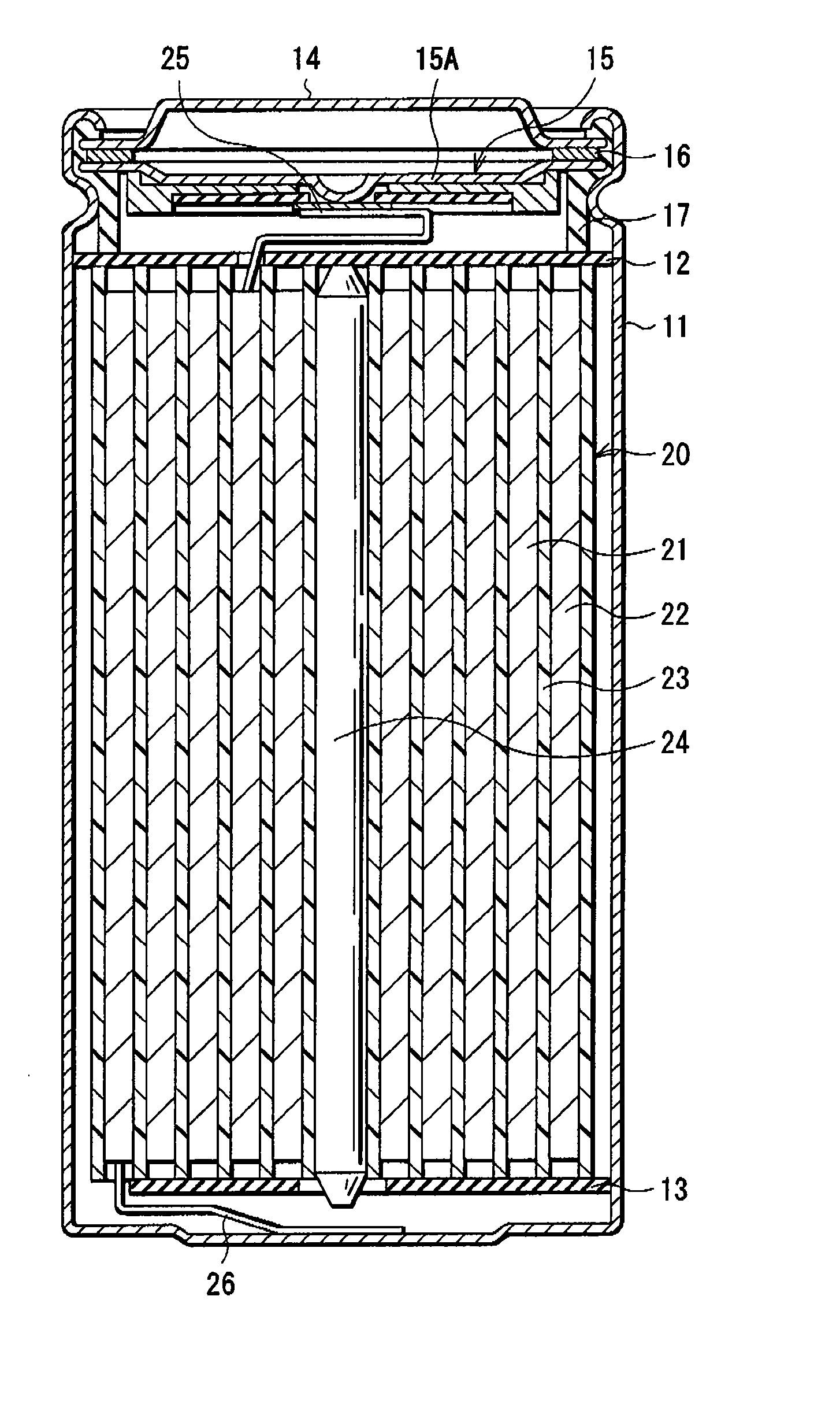
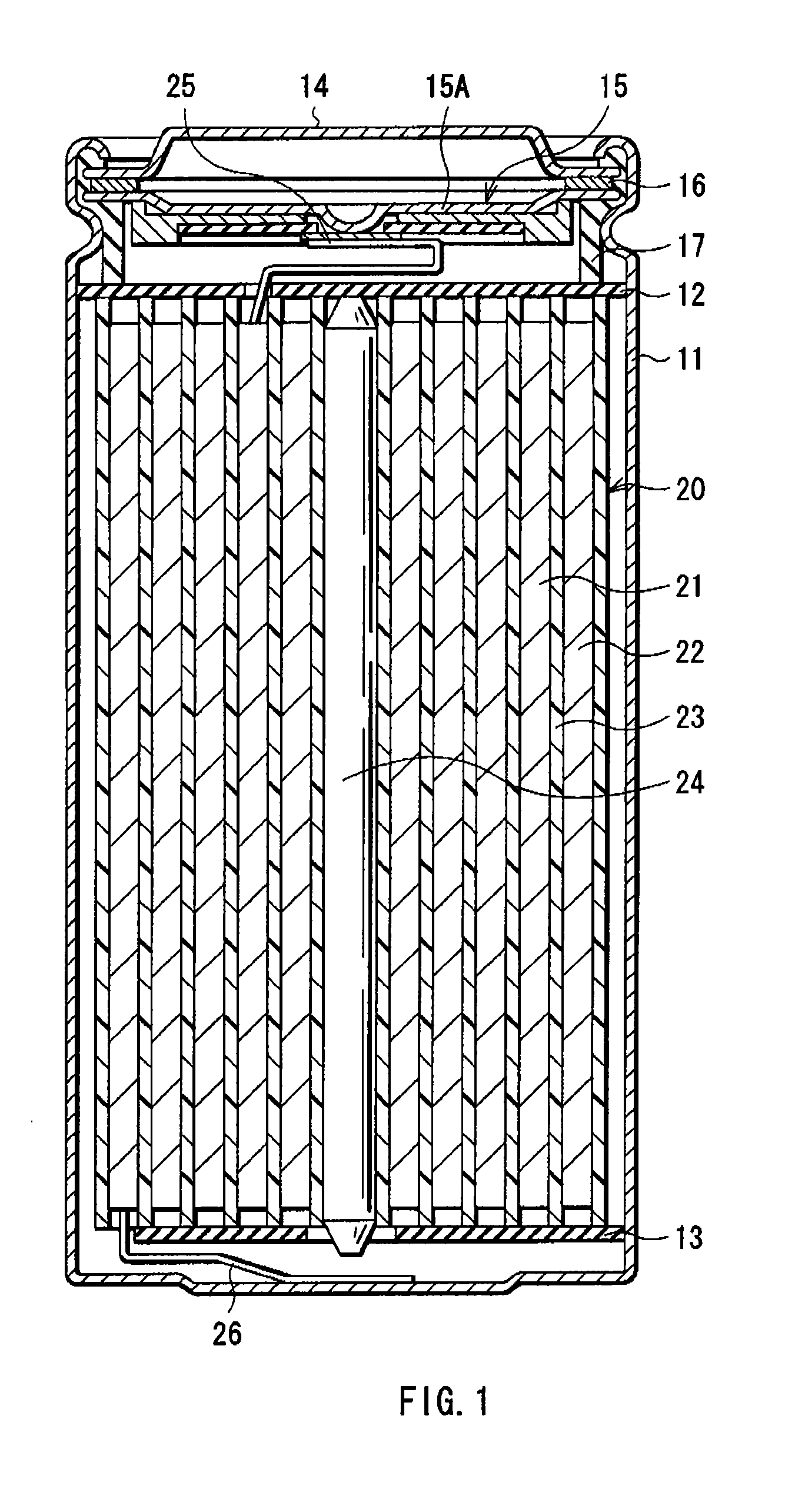
InactiveUS20080131766A1Electrolyte moving arrangementsOrganic electrolyte cellsEngineeringAlkali metal
A flat battery includes a positive electrode having voids therein, a negative electrode, a separator, an electrolyte solution, and a sealed case. The negative electrode is composed of a metal including an alkali metal and is disposed facing the positive electrode. The separator is interposed between the positive electrode and the negative electrode and electrically insulates the positive electrode from the negative electrode so that they are not brought into direct contact with each other. The electrolyte solution is impregnated into the separator and is interposed between the positive and negative electrodes. The sealed case contains the positive and negative electrodes, the separator, and the electrolyte solution. A volume of the electrolyte solution is larger than a space volume at the side of the positive electrode when space formed in the sealed case is divided by a plane dividing into two sections at the middle in the thickness direction thereof.
Owner:PANASONIC CORP
Primary high energy density balanced cell with safety circuit
InactiveUS20080070099A1Balanced stoichiometric ratioIncrease capacityNon-aqueous electrolyte cellsNon-aqueous electrolyte accumulator electrodesHigh energyManganese
A primary electrochemical cell having a capacity in excess of 0.5 AHr and a high energy density anode comprised of a metal selected from lithium, sodium, potassium, calcium and manganese and a cathode active material, wherein the discharge capacity of the anode is at least substantially equal to or in excess of the discharge capacity of the cathode active material, and wherein said cell further comprises a voltage monitor and cell circuit cut-off whereby when the voltage monitor senses a discharge voltage of about 1.6 or lower, said circuit cut-off are actuated to cut off the cell circuit to thereby prevent hazardous conditions of cell reversal or anode metal plating.
Owner:BARRELLA JOSEPH
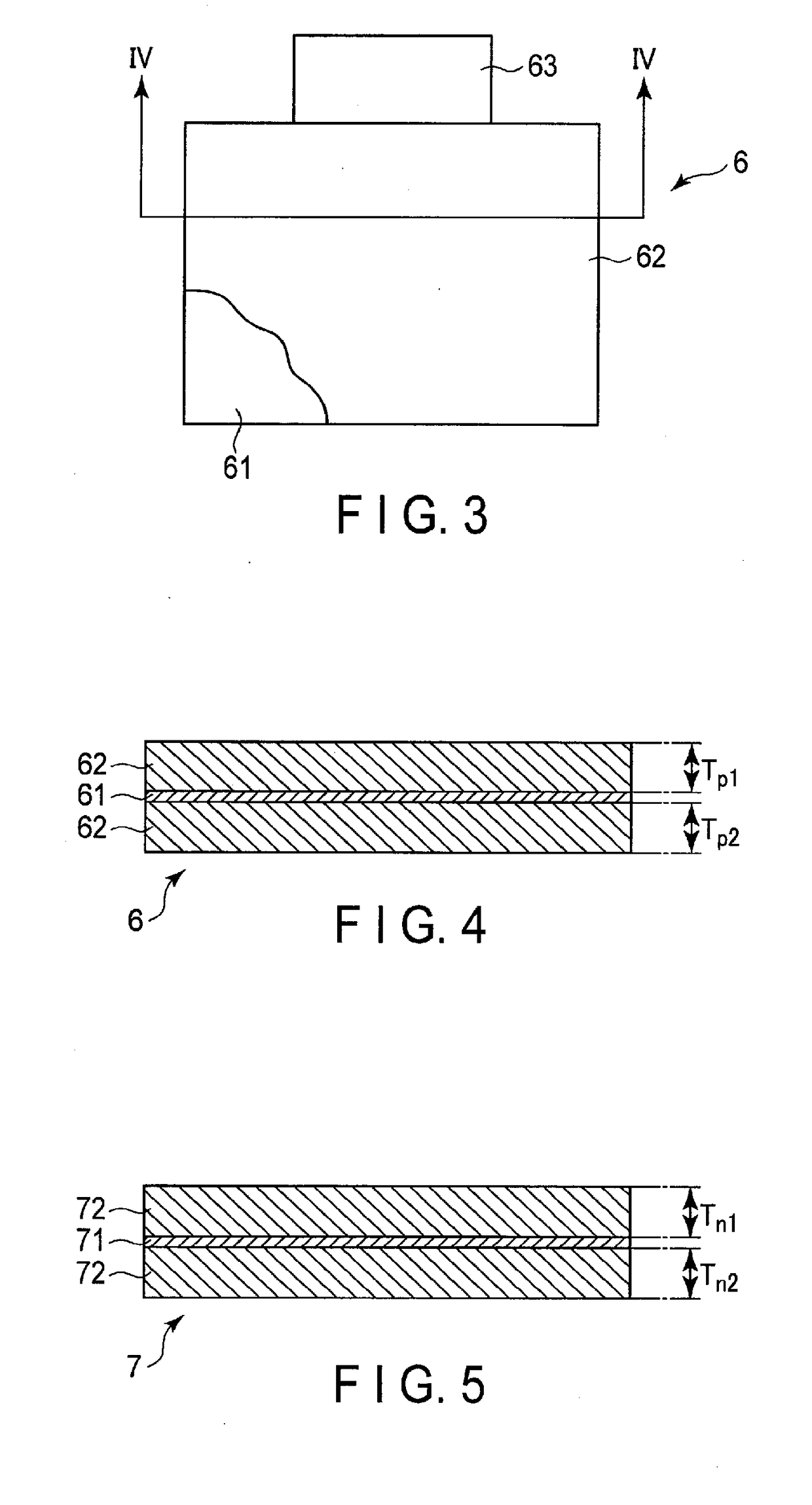
Nonaqueous electrolyte battery and battery system
According to one embodiment, there is provided a nonaqueous electrolyte battery including a positive electrode, a negative electrode and a nonaqueous electrolyte. The positive electrode includes a positive electrode active material layer including a lithium nickel cobalt manganese composite oxide. The negative electrode includes a negative electrode active material layer including a spinel type lithium titanate. The nonaqueous electrolyte has an ion conductivity of not less than 7 mS / cm and not more than 10 mS / cm at 25° C. A capacity ratio p / n is within a range of not less than 1.4 and not more than 1.8. A thickness ratio Tp / Tn is within a range of not less than 1.05 and less than 1.3. A ratio Pp / Pn is within a range of not less than 0.55 and less than 0.8.
Owner:KK TOSHIBA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com