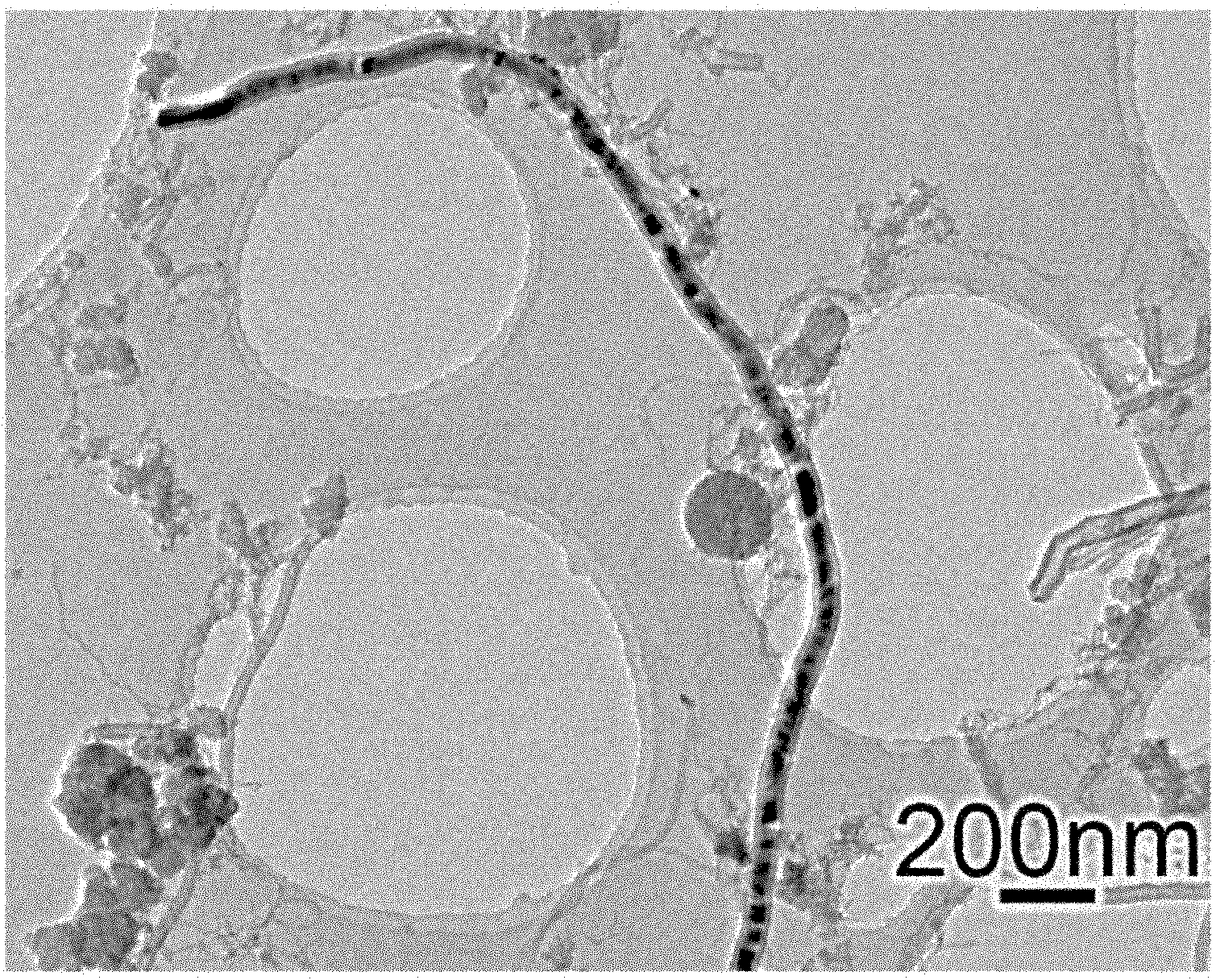
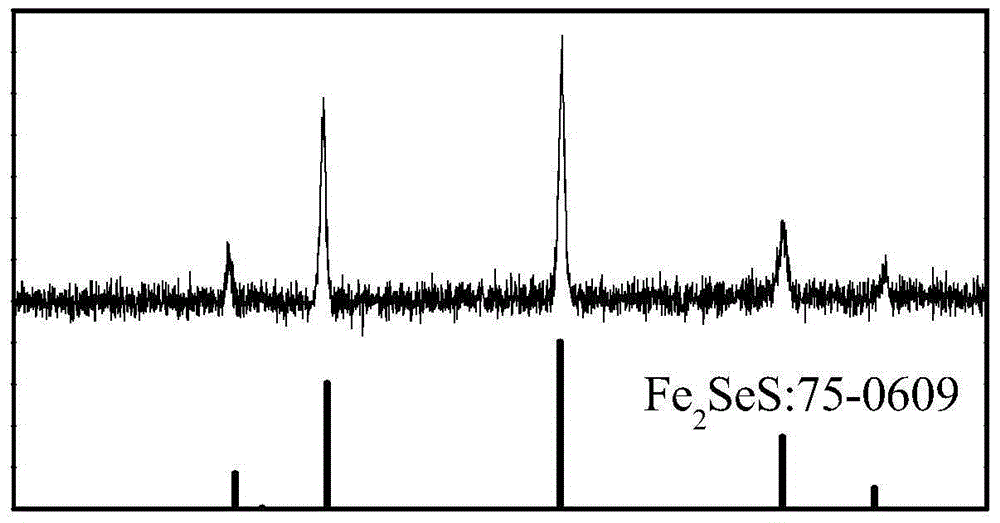
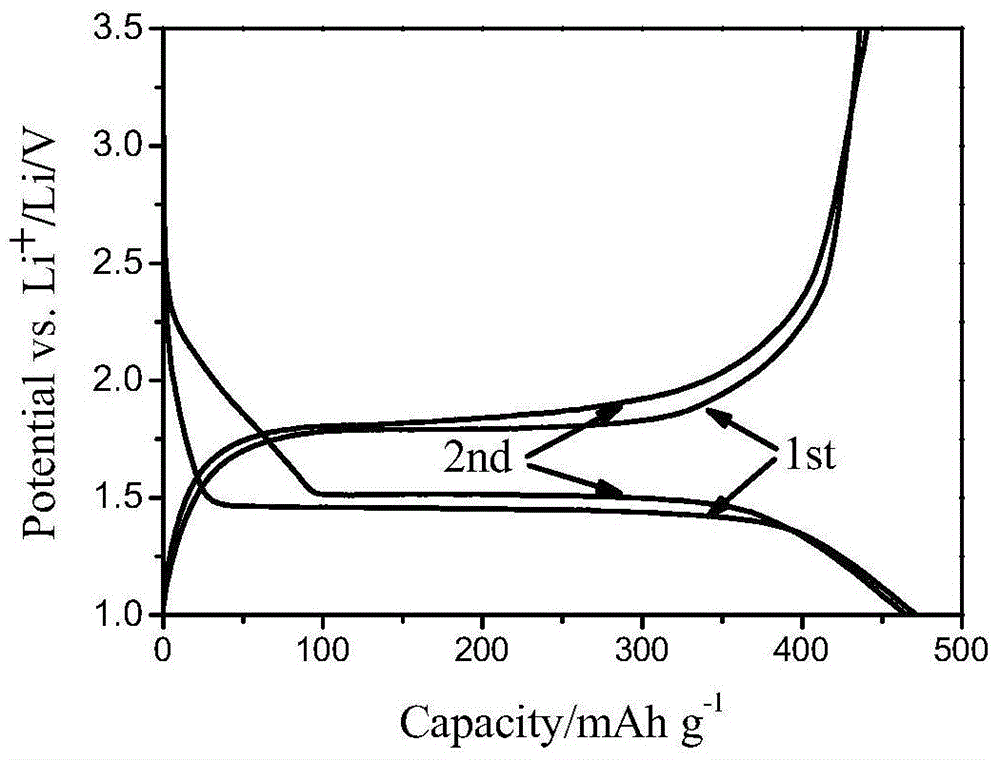
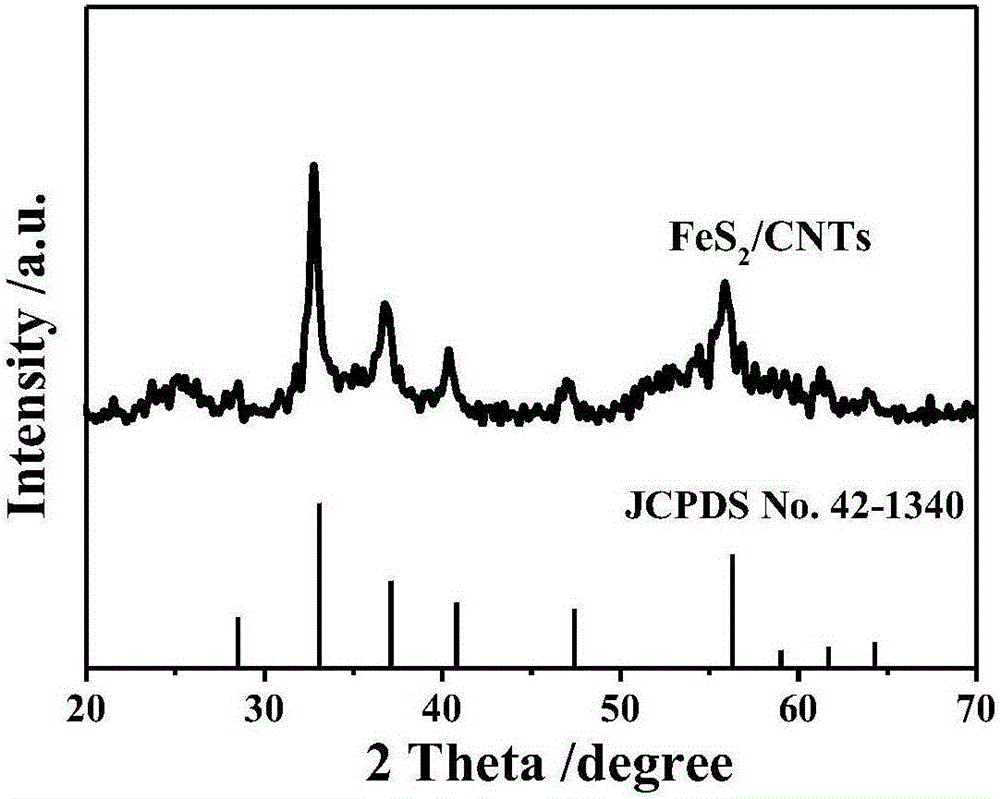
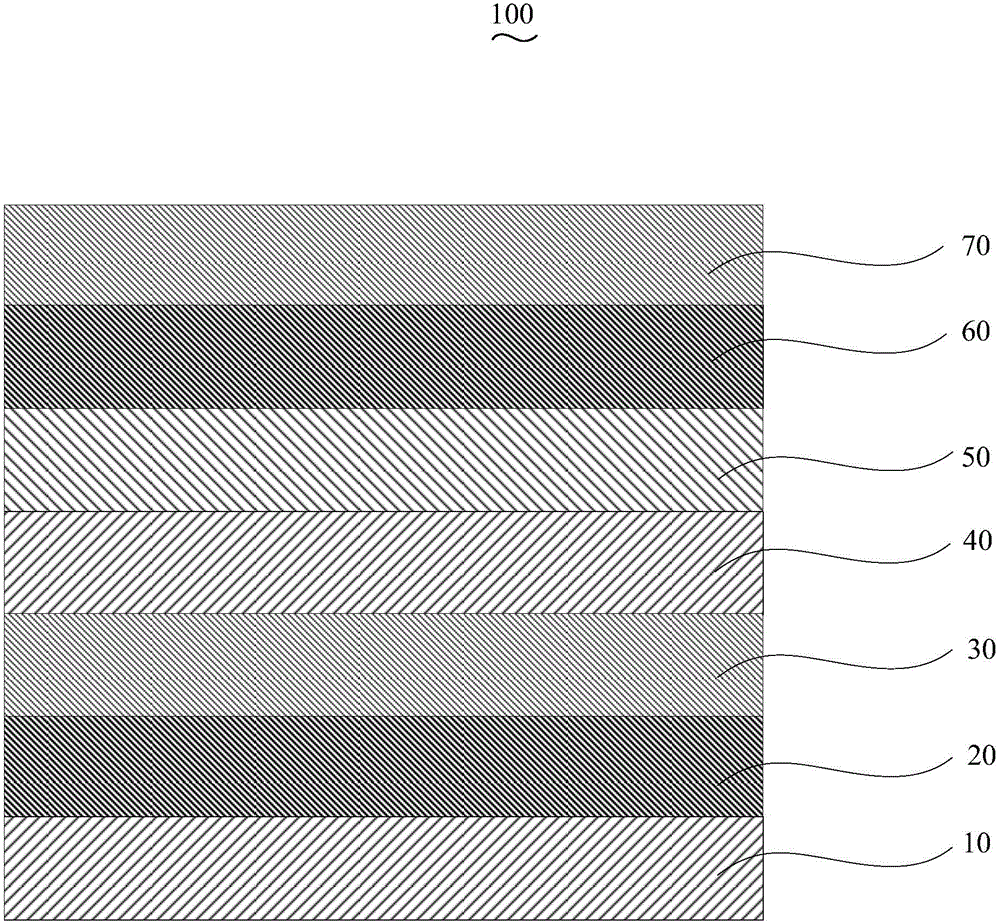
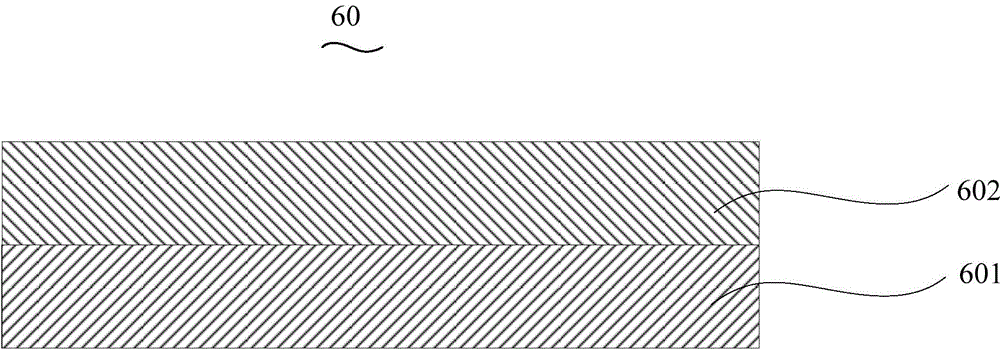
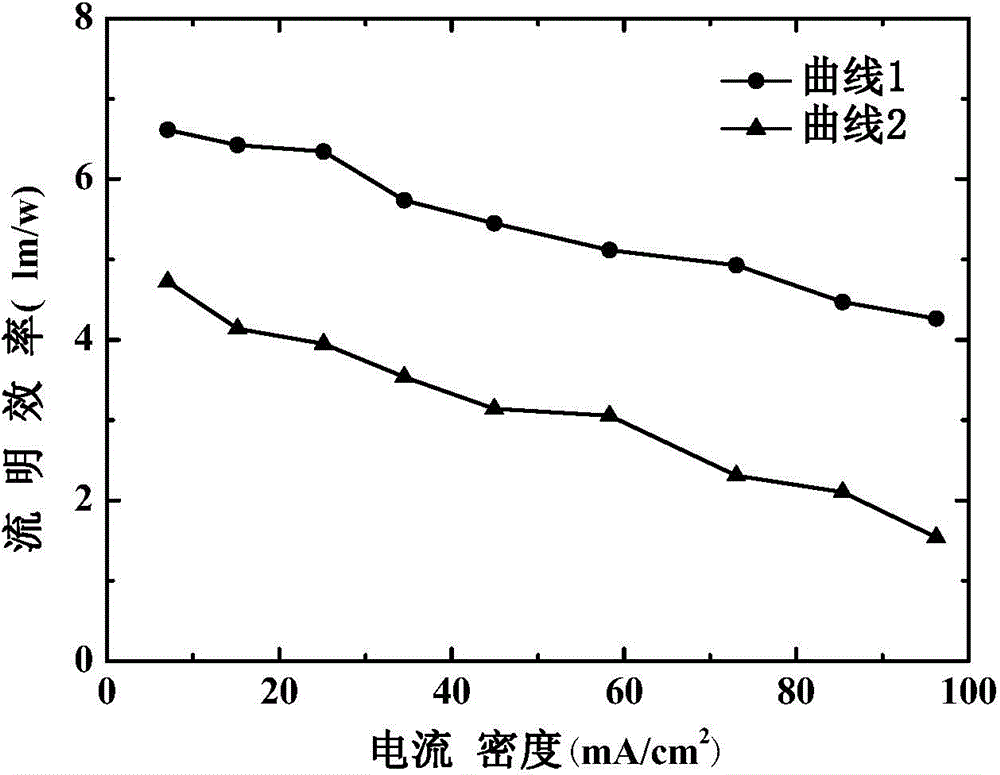
Patents
Literature
37 results about "Iron(III) sulfide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
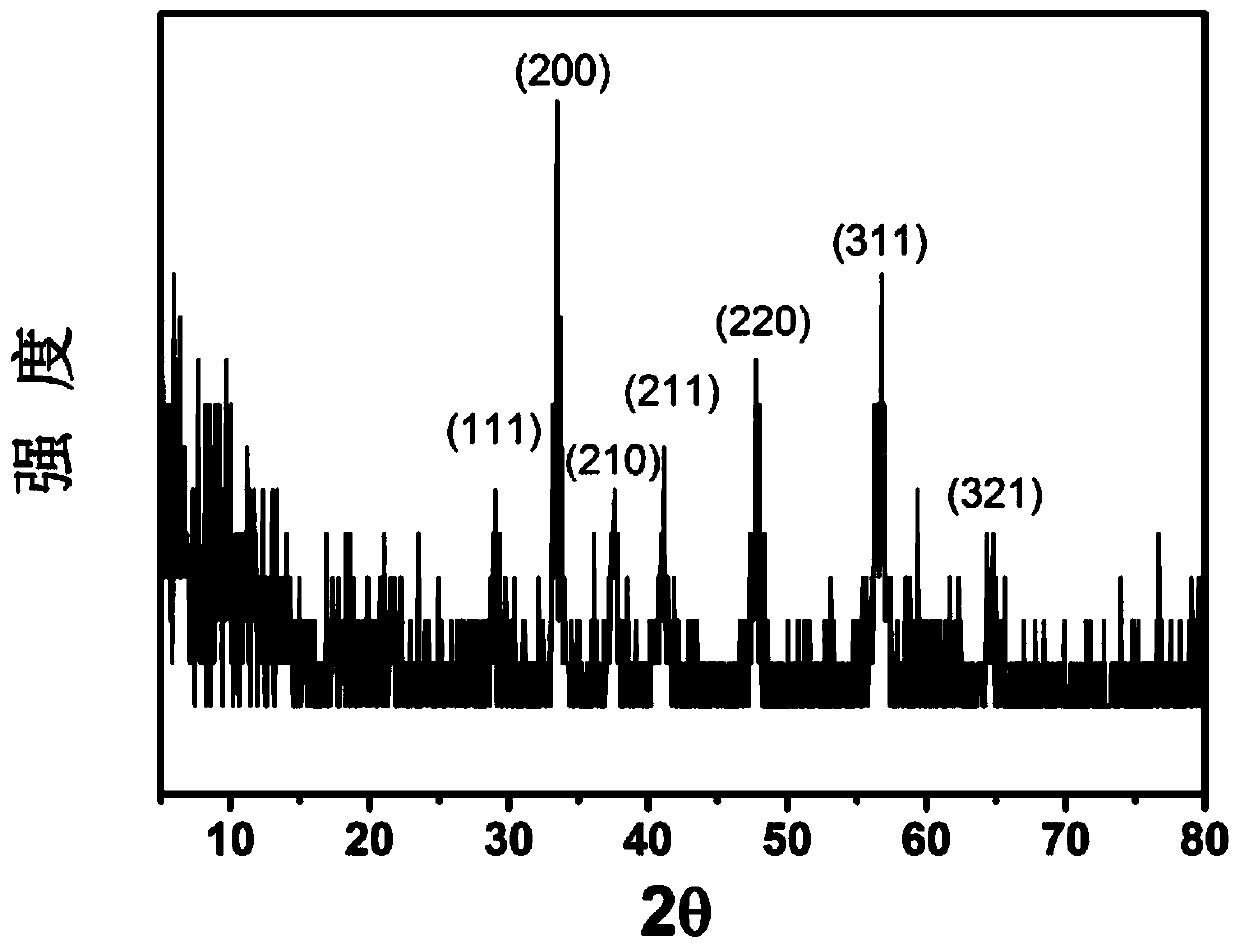
Iron(III) sulfide, also known as ferric sulfide or sesquisulfide, is one of the three iron sulfides besides FeS and FeS₂. It is a solid, black powder but decays at ambient temperature into a yellow-green powder.
High discharge capacity lithium battery
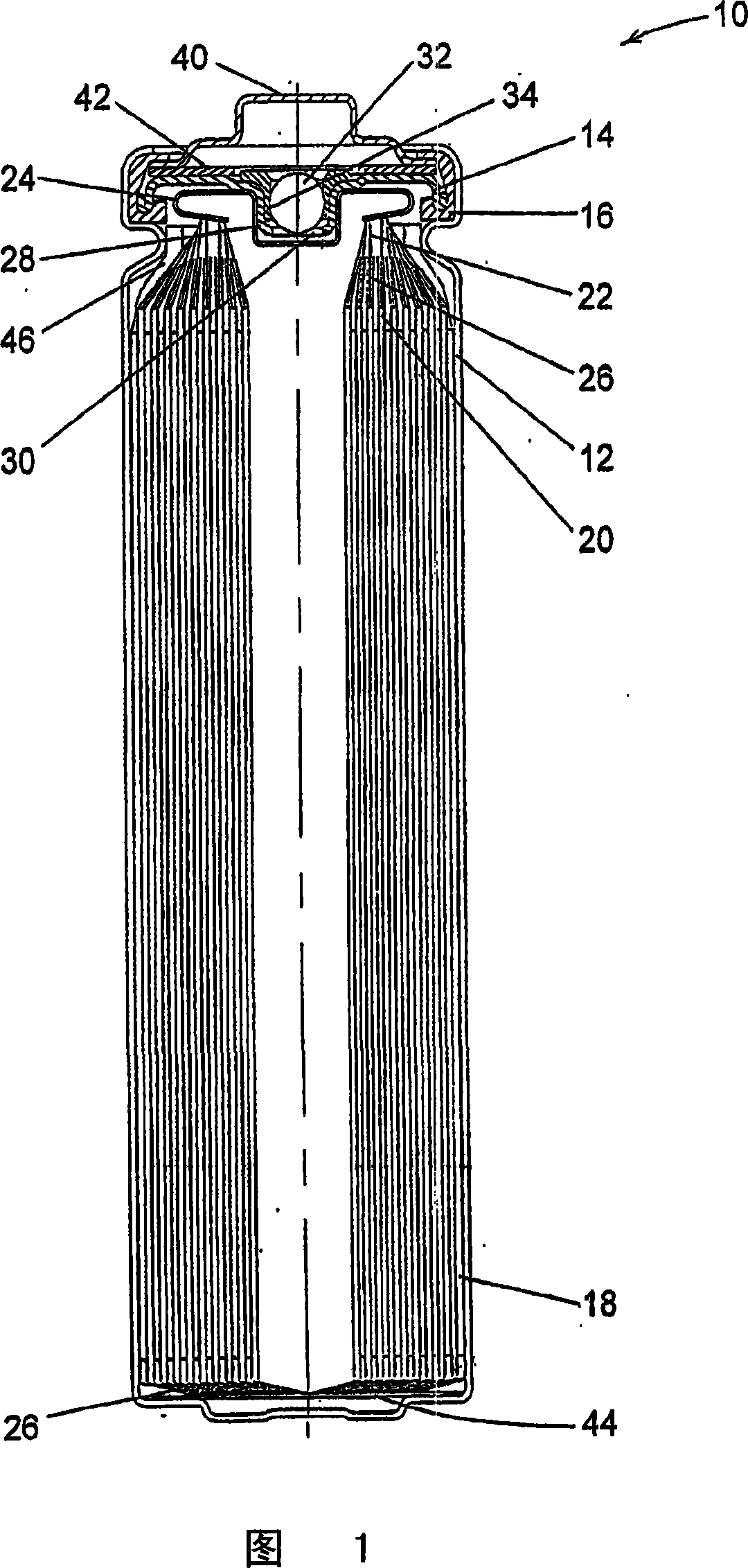
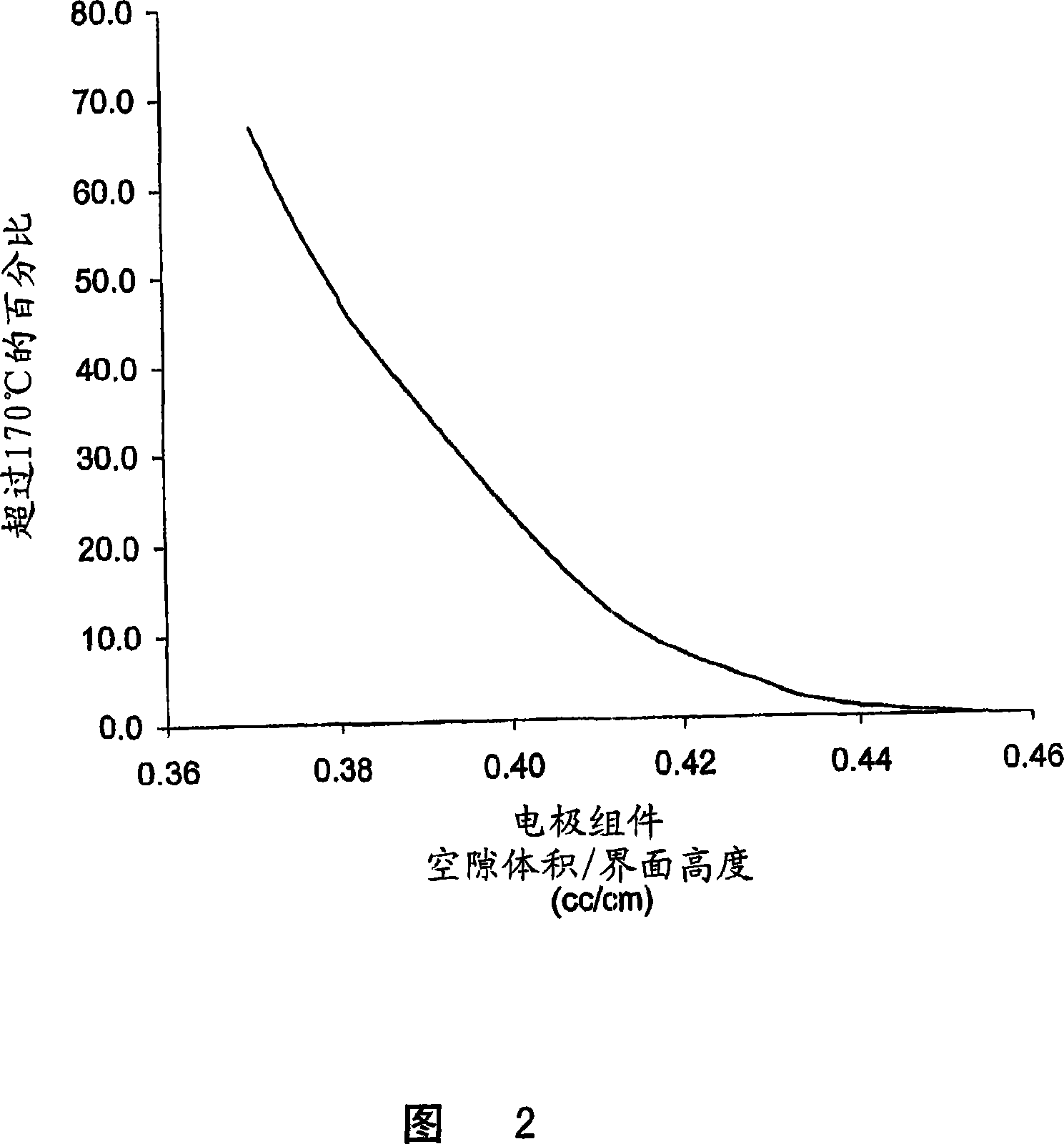
A lithium / iron disulfide electrochemical battery cell with a high discharge capacity. The cell has a lithium negative electrode, an iron disulfide positive electrode and a nonaqueous electrolyte. The iron disulfide of the positive electrode has a controlled average particle size range which allows the electrochemical cells to exhibit desired properties in both low and high rate applications. In various embodiments, the iron disulfide particles are wet milled, preferably utilizing a media mill or milled utilizing a non-mechanical mill such as a jet mill, which reduces the iron disulfide particles to a desired average particle size range for incorporation into the positive electrode.
Owner:ENERGIZER BRANDS
1.5V lithium iron disulfide button cell with metal framework positive pole
InactiveCN101202347AInhibit swellingImprove discharge capacityElectrode carriers/collectorsOrganic electrolyte cellsFiberIron(III) sulfide
The invention discloses a 1.5V lithium iron disulfide button battery with a metal framework anode. The button battery comprises a metal shell in two-half type. A septum and filling electrolyte are arranged inside the shell; two sides of the septum are provided with an anode and a cathode respectively; the septum is polypropylene and polythene film with micropores, or composite film of the polypropylene and the polythene, or polypropylene felt, or paper film with fibers or glass fiber septum. The anode consists of a current collector and an anode material which is filled in the current collector; the metal framework comprises foaming nickel, fiber nickel, foaming iron, foaming copper, foaming aluminium, foaming titanium or sintered stainless steel; the anode material consists of iron disulfide, conductive agent and binder; the cathode is metal lithium, lithium aluminium alloy or lithium silicon alloy; the electrolyte is prepared by lithium salt which is dissolved in organic solution. The invention has the advantages of restraining the expansion problem which is generated in the discharging process of the battery, simple processes and being suitable for large-scale industrial production.
Owner:SHANDONG SHENGONGHAITE ELECTRONICS TECH
Carbon nanotube composite material filled with ferrous sulfide, preparation method and application thereof
InactiveCN102010690AExcellent electromagnetic wave absorption performanceThe reaction process is simpleNanostructure manufactureWax coatingsEpoxyParaffin wax
The invention relates to a carbon nanotube composite material filled with ferrous sulfide, a preparation method and an application thereof, in particular to a carbon nanotube composite material which is filled with the ferrous sulfide and has the advantages of adjustable electrical conductivity, light weight, good stability and stronger broadband microwave absorbability, a preparation method and an application thereof. The key point of the carbon nanotube composite material filled with the ferrous sulfide is as follows: the cavity of the carbon nanotube is provided with the ferrous sulfide, wherein, the molecular formula of the ferrous sulfide is Fe7S8. In the preparation method of the carbon nanotube composite material filled with the ferrous sulfide, the Fe7S8 in the cavity of the carbon nanotube is filled in situ during the growth process of the carbon nanotube. In the invention, the carbon nanotube composite material is taken as an electromagnetic wave absorbing material and an electromagnetic shielding material; and when in use, the carbon nanotube composite material is uniformly dispersed in paraffin wax or epoxy resin and then coated on the surface of an object requiring for being coated.
Owner:ZHEJIANG NORMAL UNIVERSITY
Preparing method and application of lithium ion battery cathode material selenium ferrous sulfide
InactiveCN104485455AHigh specific capacityImprove cycle performanceCell electrodesIron(III) sulfideIron powder
The invention discloses a preparing method and application of lithium ion battery cathode material selenium ferrous sulfide. The method includes the steps that iron powder, selenium powder and sulfur powder are subjected to mixed target pressing; under the protective atmosphere of argon, selenium ferrous sulfide is sintered through a tube furnace; ball grinding is performed. The prepared selenium ferrous sulfide is used as a lithium ion battery cathode material. The preparing method is easy to operate, simple in process and suitable for large-scale production. The selenium ferrous sulfide cathode material is high in specific capacity, circulating performance and rate performance and is a lithium ion battery cathode material high in performance.
Owner:INST OF ELECTRONICS ENG CHINA ACAD OF ENG PHYSICS
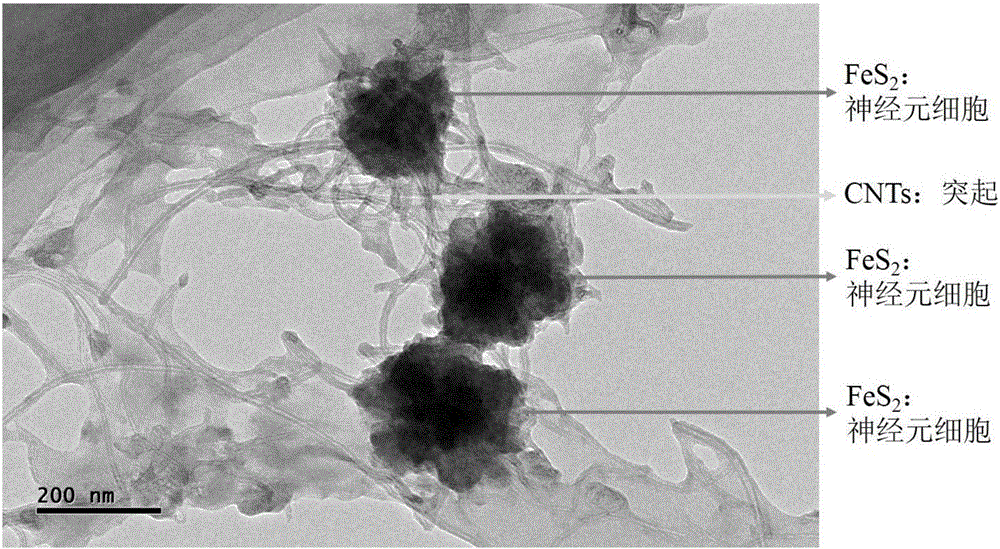
Iron disulfide/carbon nano tube composite material of neural network structure and preparation method thereof
InactiveCN106848230AImprove cycle performanceImprove conductivityCell electrodesSecondary cellsIron(III) sulfideCarbon nanotube
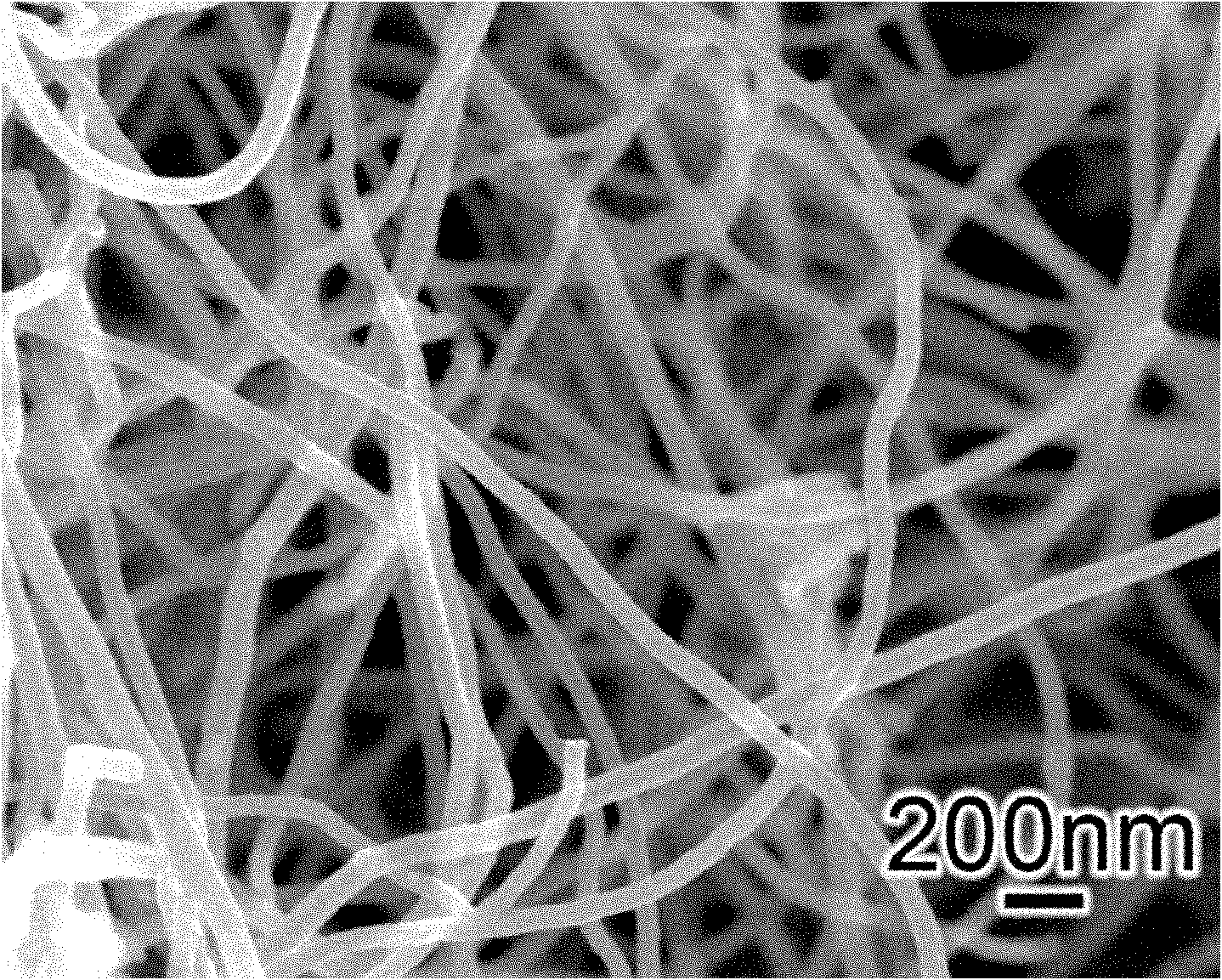
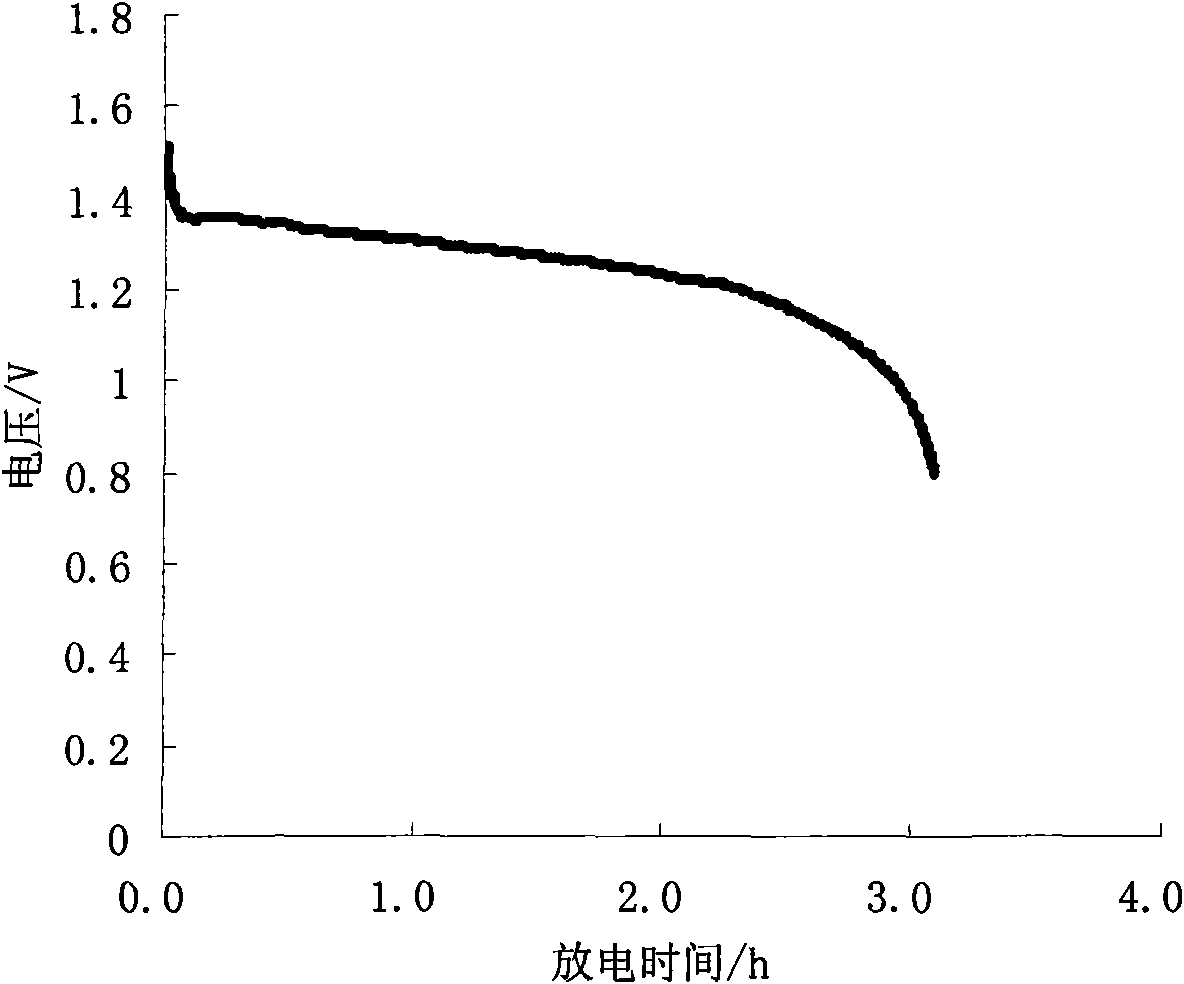
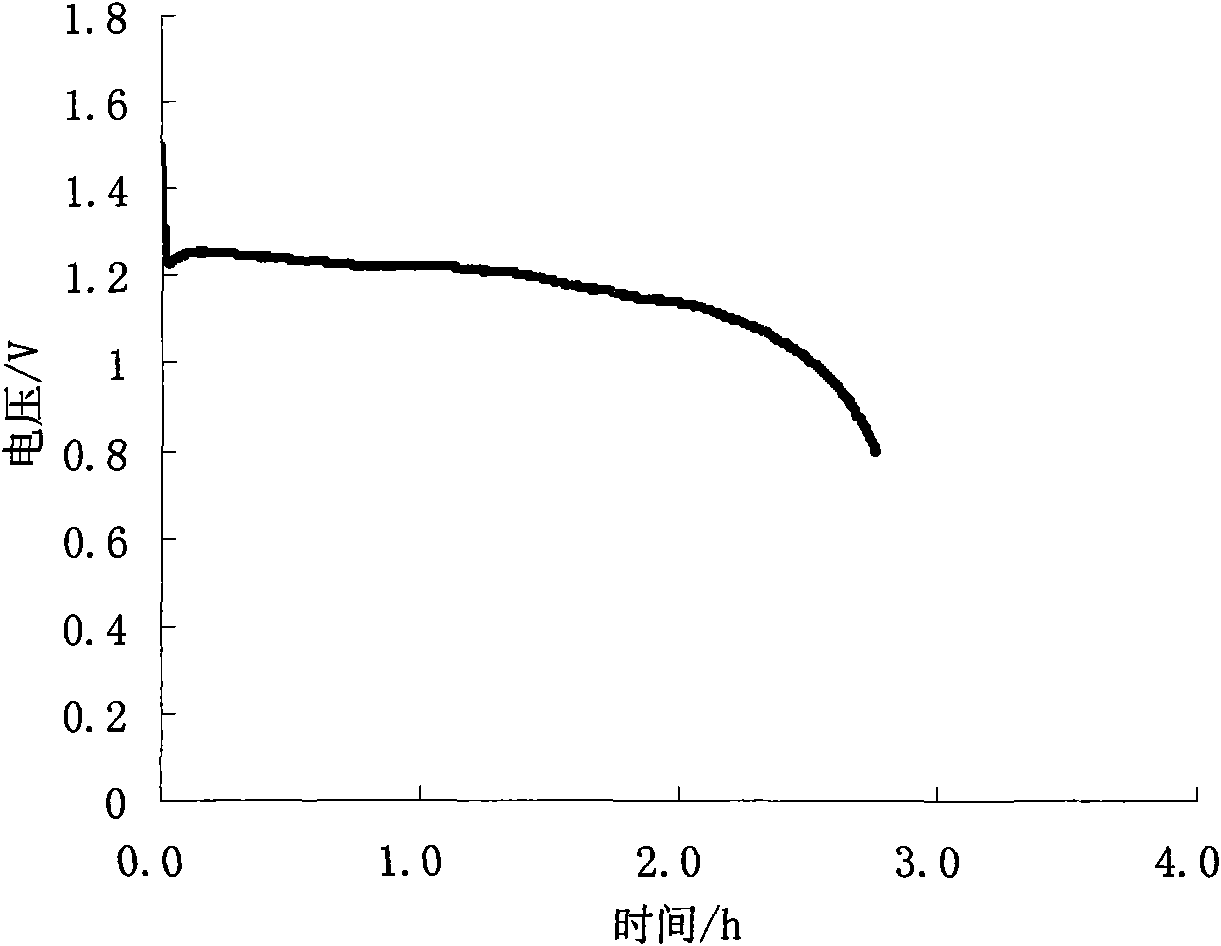
The invention provides an iron disulfide / carbon nano tube composite material of a neural network structure and a preparation method thereof. The preparation method comprises the following steps of adding a carbon nano tube into mixed liquor of ethylene glycol and N,N'-dimethylformamide, and carrying out ultrasonic treatment to obtain carbon nano tube uniformly dispersed mixed liquor; adding ferrous sulfate, sublimed sulfur and urea into the mixed liquor, sufficiently agitating an obtained mixture, and then transferring the mixture into a hydrothermal reaction kettle to carry out a reaction; centrifugally separating a deposit, washing the deposit by using deionized water and alcohol, and then carrying out vacuum drying to obtain the iron disulfide / carbon nano tube composite material which is of the neural network structure. The iron disulfide / carbon nano tube composite material is of a three-dimensional neural network structure which is formed by mutually connecting neurons for which an iron disulfide particle is used as a neuron cell and the carbon nano tube is used as a prominence, wherein the diameter of the iron disulfide particle is not more than 200nm. The neural network structure can be used for improving the electric conductivity of a material; meanwhile, the volume expansion of iron disulfide in charging and discharging processes is buffered through the mechanical property of the carbon nano tube; besides, the wettability of an electrolyte on the material can be also improved through the three-dimensional structure of a neutral network and the nano size of the iron disulfide; the cyclical stability of an iron disulfide material as a sodium-ion negative electrode is greatly improved.
Owner:TIANJIN UNIV
No-lag lithium / iron disulfide round cell and manufacturing method thereof
InactiveCN101577334ADelay EliminationImprove stabilityElectrode manufacturing processesElectrode carriers/collectorsIron(III) sulfideBarium titanate
The invention provides a no-lag lithium / iron disulfide round cell and a manufacturing method thereof. The positive pole of the cell is added with a titanium compound in a certain proportion such as one or a mixture of more in an arbitrary ratio of lithium titanate, sodium titanate, barium titanate, ilmenite, titanium dioxide, and the like; the titanum compound, iron disulfide, a conductive agent and a binder are evenly mixed and stirred to be made into slurry, and then, the slurry is coated on an aluminium substrate, and the aluminum substrate is milled and cut into a positive pole sheet; a lithium or lithium alloy negative pole is combined with the positive pole sheet. The lithium / iron disulfide round cell is made after adding the electrolyte. The cell can be widely applied in various fields. The technical method has simple process, eliminates the delay phenomenon in the discharge course, and enhances the stability of cell discharge voltage.
Owner:SHANDONG SHENGONGHAITE ELECTRONICS TECH
Method for recycling ferric sulphide-containing tailings
ActiveCN105070937AHigh recovery rateLow running costFinal product manufactureBiochemical fuel cellsIron(III) sulfidePollution
The invention relates to a method for recycling ferric sulphide-containing tailings. According to the method, oxidizing half-reaction FeS2+8H2O -> Fe<3+>+16H<+>+2SO4<2->+15e<-> and reducing half-reaction 3.75O2+15H<+>+15e<-> -> 7.5H2O (3) are respectively put into an anode chamber and a cathode chamber of a microbial fuel cell (MFC) for microbial leaching, so as to obtain ferric sulphide-containing tailings; and when metal is recovered, electrons released by oxidizing half-reaction can also be recovered by an outer circuit of the MFC in a form of electric energy, so that the operation cost is reduced. In addition, protons generated by oxidizing half-reaction are transmitted to a cathode to participate into reduction reaction to generate water, and are continuously consumed, so that the leaching rate can be significantly improved to improve the metal recovery rate; meanwhile, the generated water is environment-friendly and free of secondary pollution; and corrosion to equipment caused by acid production can also be retarded.
Owner:JIANGNAN UNIV
Process and Reagent for Removal of Oxygen from Hydrocarbon Streams
A method of using a sulfided iron reagent to remove oxygen from gaseous and liquid fluid streams such as natural gas, light hydrocarbon streams, crude oil, acid gas mixtures, carbon dioxide gas and liquid streams, anaerobic gas, landfill gas, geothermal gases and liquids, and the like is disclosed. In a preferred embodiment, the reagent is made by mixing, agglomerating and shaping finely powdered ferrous carbonate, preferably siderite which are used to remove oxygen from a hydrocarbon or carbon dioxide stream that also contains sulfur compounds such as hydrogen sulfide.
Owner:NEW TECH VENTURES
Selective recovery of minerals by flotation
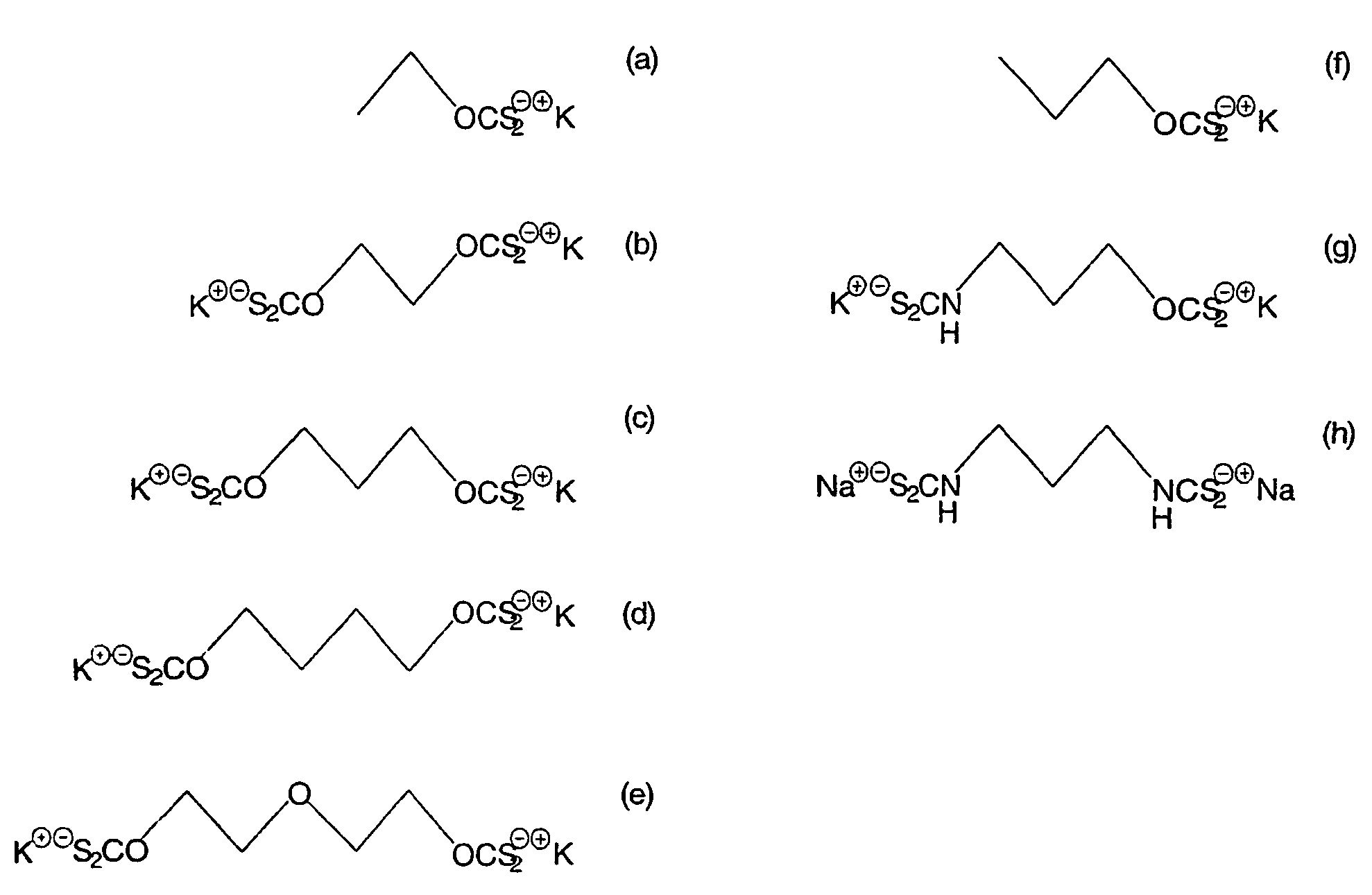
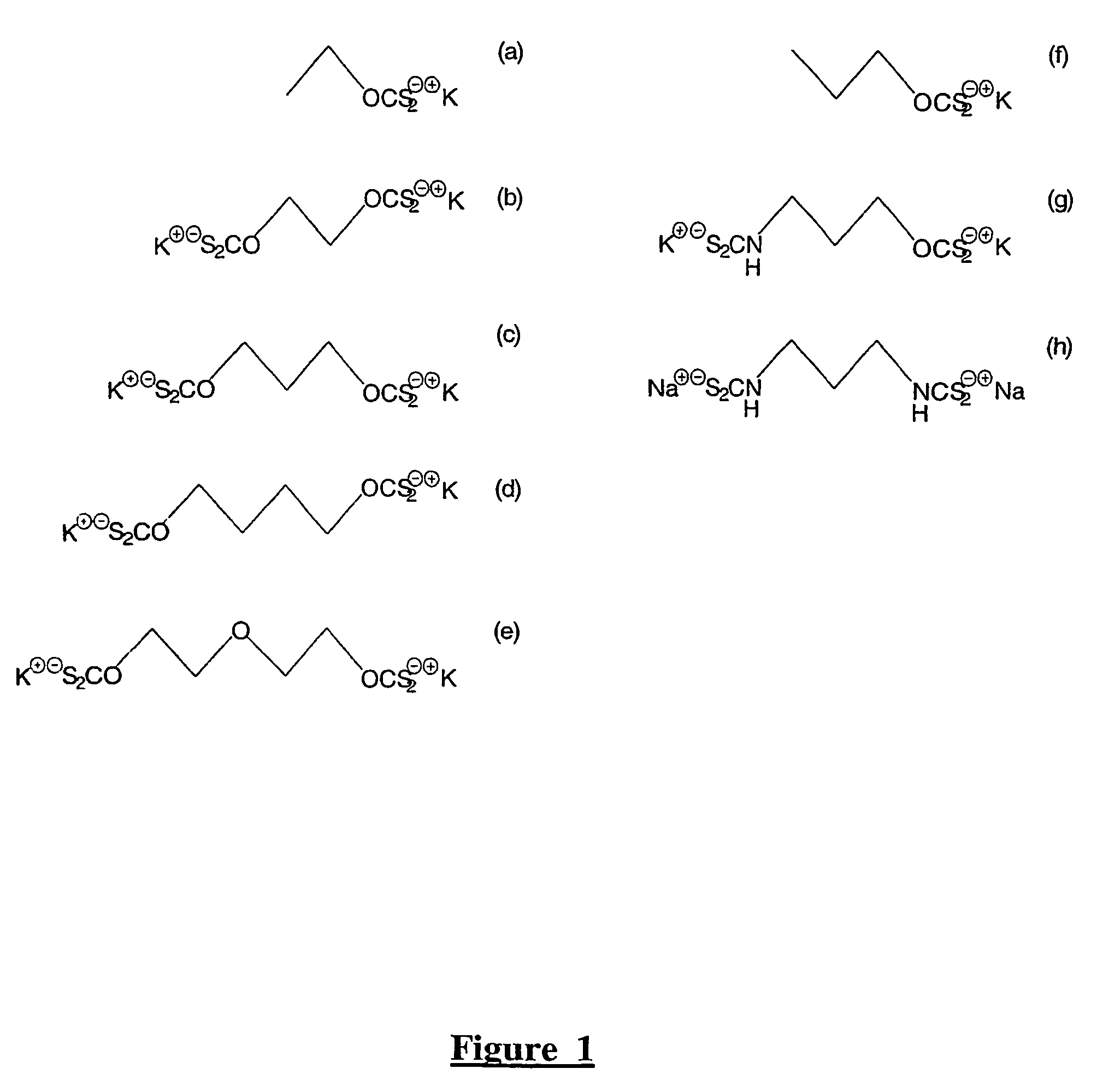
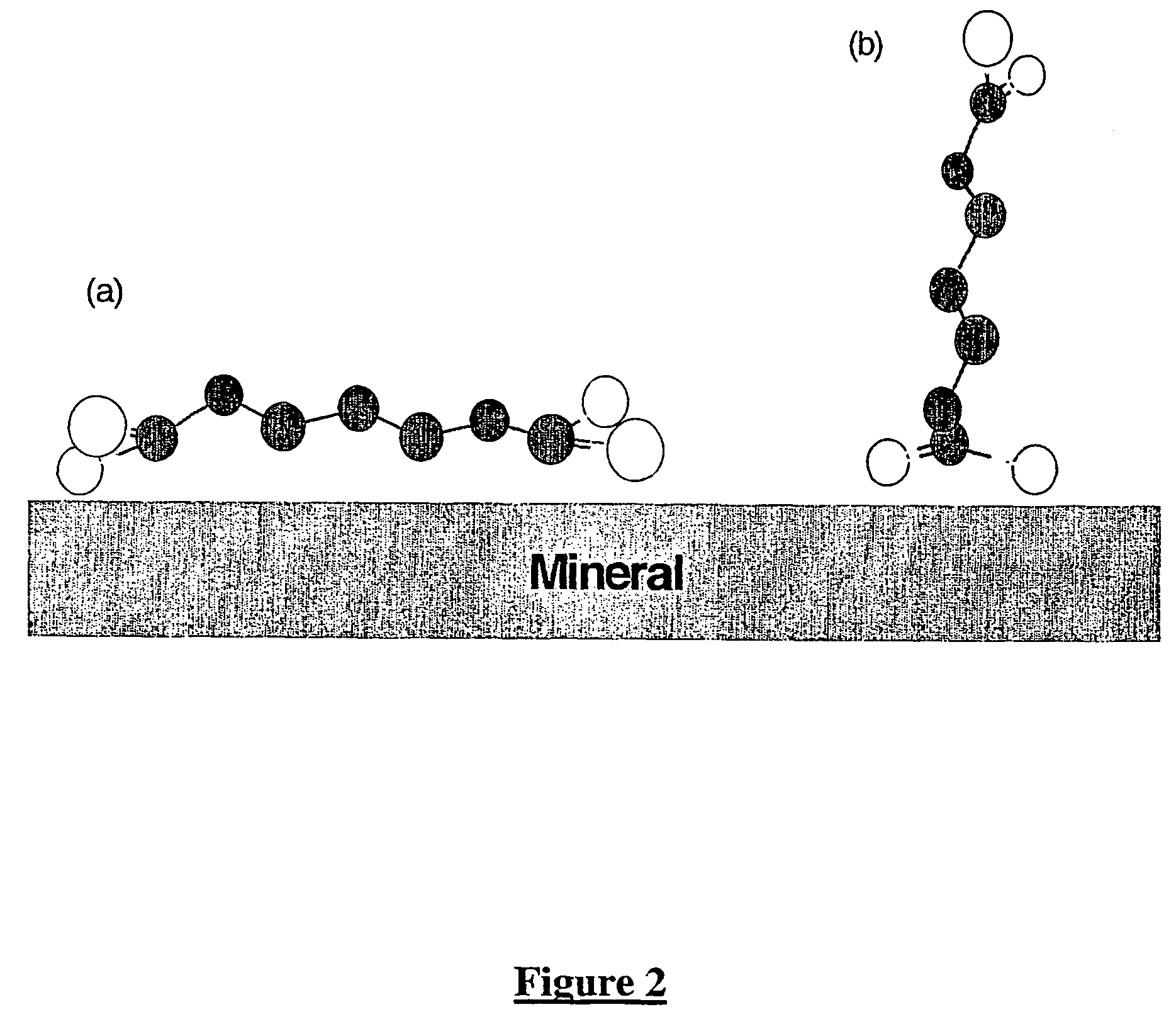
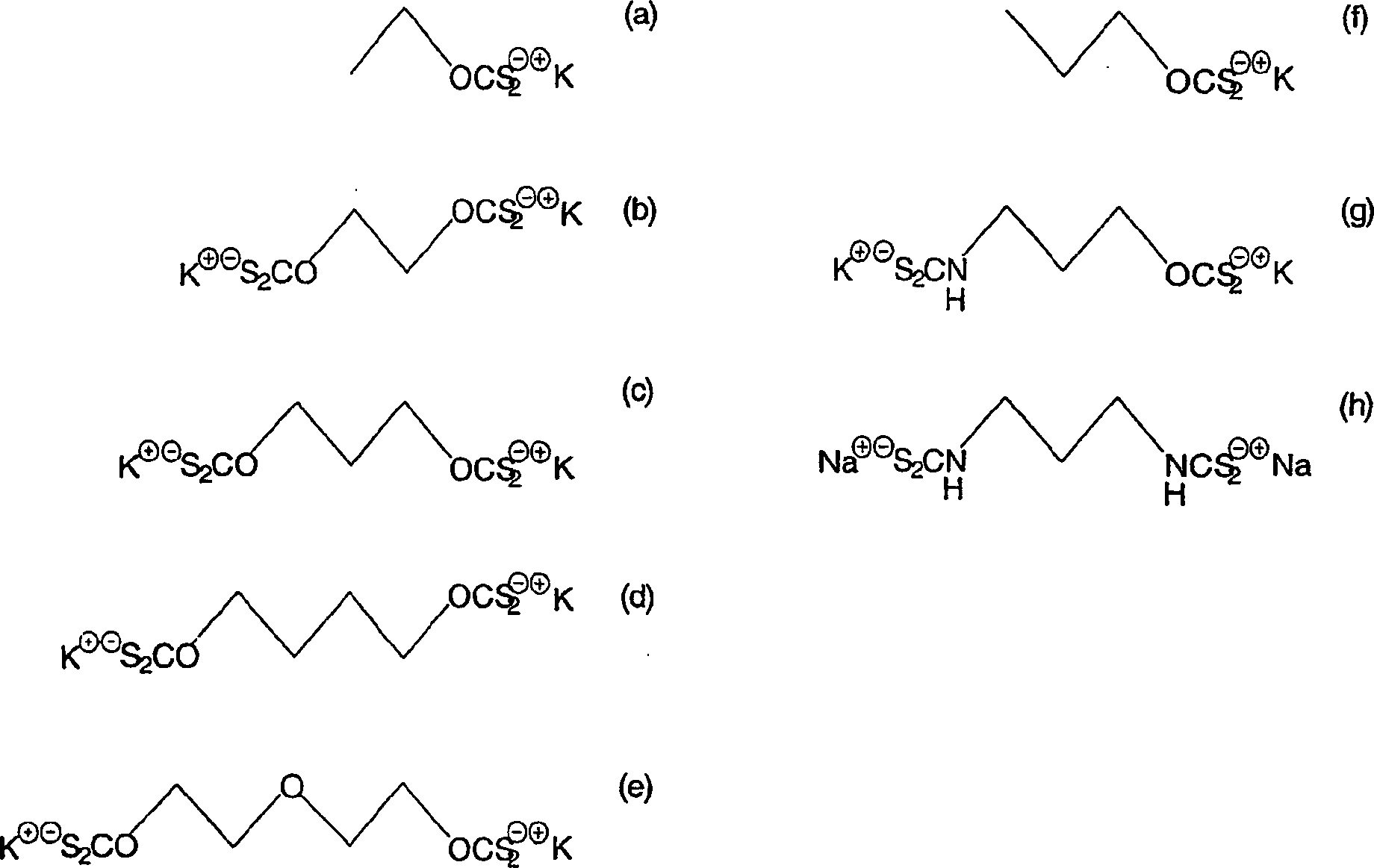
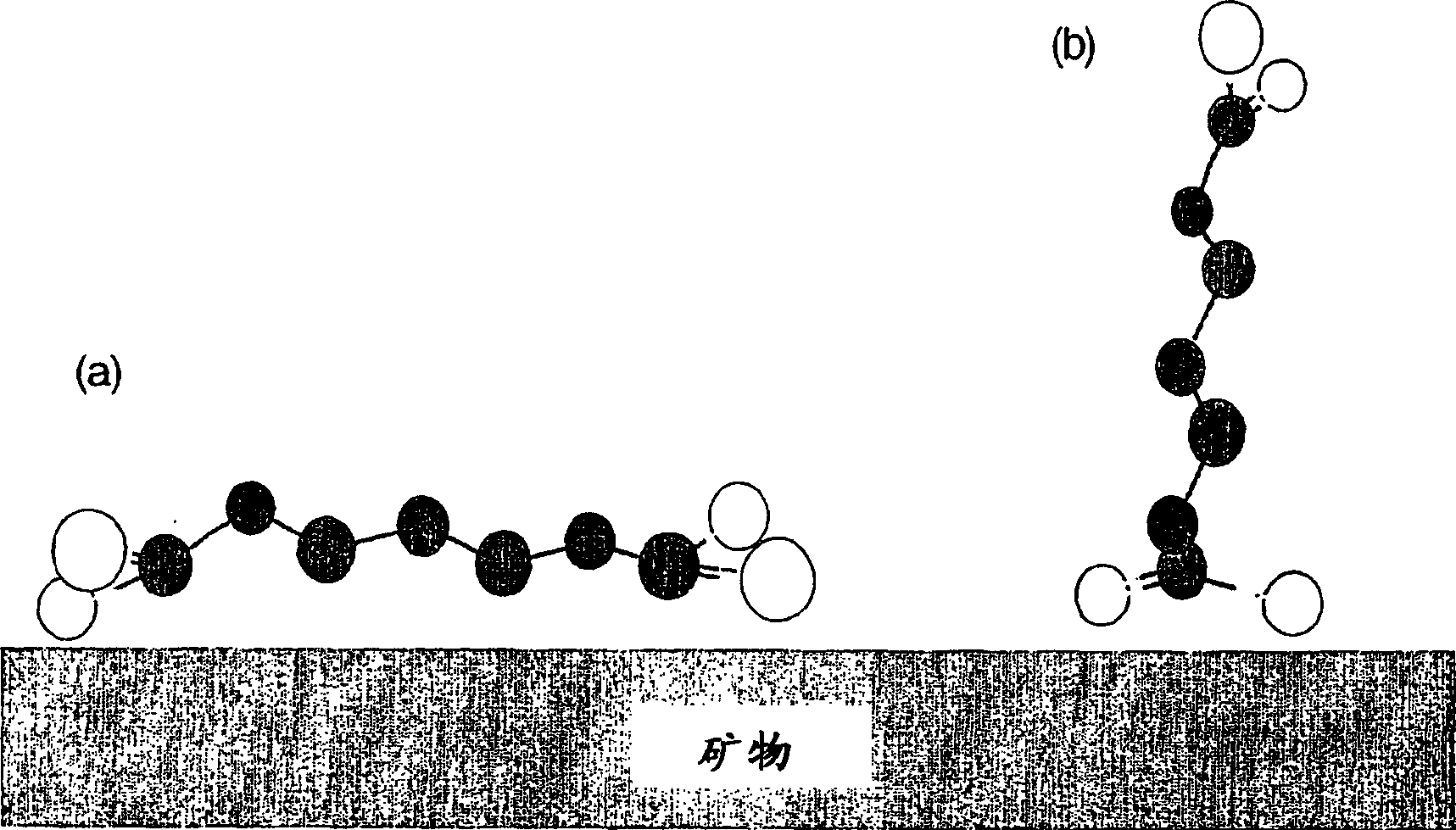
A method of recovering a target mineral from an ore containing the target mineral and an iron sulphide mineral comprising the steps of: a) grinding the ore to liberate target mineral from the iron sulphide mineral; b) forming a pulp of said ore; c) selecting a collector having the structure as follows: X—R—Y where R is a branched or straight chain hydrophobic hydrocarbon or polyether chain, and X and Y represent metal coordinating functional groups, d) add the collector to the pulp at a concentration at which the target mineral is able to be floated in preference to the iron sulphide mineral; and e) subjecting the pulp to froth flotation. The metal coordinating sulphur based functional groups may be identical or different.
Owner:COMMONWEALTH SCI & IND RES ORG
Selective recovery of minerals by flotation
A method of recovering a target mineral from an ore containing the target mineral and an iron sulphide mineral comprising the steps of: a) grinding the ore to liberate target mineral from the iron sulphide mineral; b) forming a pulp of said ore; c) selecting a collector having the structure as follows: X-R-Y where R is a branched or straight chain hydrophobic hydrocarbon or polyether chain, and X and Y represent metal coordinating functional groups, d) add the collector to the pulp at a concentration at which the target mineral is able to be floated in preference to the iron sulphide mineral; and e) subjecting the pulp to froth flotation. The metal coordinating sulphur based functional groups may be identical or different.
Owner:COMMONWEALTH SCI & IND RES ORG
Gray ceramic pigment and preparation method thereof
InactiveCN110204927AImprove high temperature stabilityHigh color strengthInorganic pigment treatmentIron(III) sulfideAcid washing
The invention discloses a gray ceramic pigment and a preparation method thereof. The gray ceramic pigment is a wrapping type pigment obtained by wrapping iron sulfide with zirconium silicate; the preparation method comprises the following steps that an iron source and a zirconium source are dissolved into water to form a solution A; the sulfide and inorganic alkali are dissolved into water to forma solution B; the solution A and the solution B are evenly mixed in a titration mode, and pH is controlled to be 8.5-9.5; after titration is completed, white carbon black is added, filtering, washing, drying, calcination, acid washing, washing and drying are performed to obtain the gray ceramic pigment obtained by wrapping iron sulfide with zirconium silicate. A liquid phase co-precipitation method is adopted for forming the gray ceramic pigment of the core-shell structure adopting iron sulfide as a core and adopting zirconium silicate as a wrapping layer , the whole pigment is gray and bright in color, the light emitting strength is remarkably improved, and the high temperature stability of the pigment is greatly improved. The preparation method is simple in technology, the product hightemperature stability is high, raw materials are cheap, the production cost is low, and therefore the pigment can be industrially used, applied and popularized.
Owner:FOSHAN HUAYI CERAMIC COLORS CO LTD
Method for preparing nano ferrous disulfide microsphere with limited range
InactiveCN110182851AUniform particlesSimple methodCell electrodesNanotechnologyVulcanizationIron(III) sulfide
The invention discloses a method for preparing nano ferrous disulfide microsphere sodium-ion battery cathode material with a limited range. The method comprises the following steps: 1) synthesizing ferriferrous oxide nanoparticles by using a high-temperature solvent method; 2) assembling ferriferrous oxide microspheres by using an emulsion method, and carrying out high-temperature surface oleic acid carbonization so as to form carbon shells; and 3) pre-etching the ferriferrous oxide microspheres, and carrying out one-step vulcanization, so as to obtain the nano ferrous disulfide microsphere sodium-ion battery cathode material. Granules of the nano ferrous disulfide microspheres prepared by using the method are all of a nano grade, each granule is uniformly coated by the carbon shell, and the nano ferrous disulfide microspheres are excellent in rate capability and cycle performance when being applied to the cathode of a sodium-ion battery.
Owner:佛山市格瑞芬新能源有限公司
Method for controlling morphology of sulfide in low-carbon high-sulfur easy-cutting steel
InactiveCN110129516AStable productionProcess efficiency improvementElectric furnaceIron(III) sulfideSlag
The invention discloses a method for controlling the morphology of a sulfide in low-carbon high-sulfur easy-cutting steel. The method comprises the following steps that (1) an electric furnace is usedfor smelting, and the steel tapping C mass percentage and the tapping temperature are controlled; (2) silicon manganese and low-carbon ferromanganese are added for deoxidizing, ferric sulfide is added to adjust a sulfur component, lime is added, and the electric furnace is controlled to reach the initial oxygen content 100-200ppm of a refining furnace after alloying; (3) ferrosilicon powder and adeoxidation slag former are used for diffusion deoxidation in refining, the mass percent of Si is controlled to be less than or equal to 0.03%, the mass percentage of Mn is adjusted to be 1.05%-1.33%, the mass percent of S is adjusted to be 0.30%-0.38%, and the oxygen content is controlled to reach 70-120 pm; and (4) after casting, rolling and forming are carried out. According to the method, theinitial oxygen content and the molten steel component after tapping steel alloying are more easily controlled, so that the length-width ratio of the morphology of the sulfide is controlled, and the condition that the ratio is larger than 1 and is less than or equal to 3 is up to 90%.
Owner:NANJING IRON & STEEL CO LTD
Sulfur-doped algae and iron composite material and preparation method and application thereof
ActiveCN107930584AOvercoming various technical difficulties faced by high-value utilizationQuick buildOther chemical processesWater contaminantsIron(III) sulfideTherapeutic effect
The invention relates to a method in the technical field of environmental protection, and specifically relates to a sulfur-doped algae and iron composite material and a preparation method and application thereof. The sulfur-doped algae and iron composite material is obtained by reducing iron modified algae based carbon under the effects of an iron source and a sulfur source. The prepared sulfur-doped algae and iron composite material can be applied to water pollution control, soil (sediment) improvement or polluted environment repair. Through the technical scheme, the limitation that the treatment effect is non-ideal, which is caused by agglomeration of a zero-valent iron material or a ferric sulfide material formerly is eliminated, various technical problems for algae biomass high-valuedutilization can be effectively overcome, and a reactor which is low in capital cost and can be quickly constructed is invested in advance.
Owner:YANTAI INST OF COASTAL ZONE RES CHINESE ACAD OF SCI
Beneficiation method of synchronously removing phosphorus and sulfur for high phosphorus sulfur iron ore
ActiveCN109759244AIncrease the density of calcium active particlesImprove iron gradeFlotationHigh concentrationIron(III) sulfide
The invention discloses a beneficiation method of synchronously removing phosphorus and sulfur for high phosphorus sulfur iron ore, and belongs to the technical field of beneficiation. The method comprises the following steps that high phosphorus sulfur iron ore is ground, the pH value of ore pulp is conditioned, and a surfactant calcium hypochlorite is added into the high concentration pulp system; the pulp is concentrated and filtered so as to eliminate disadvantageous influence of residual ions, after the concentration of the ore pulp is lowered, starch and sodium lignin sulfonate are addedto serve as a combined inhibitor of iron ore, a phosphorus sulfur synchronous removal reverse flotation test comprising one roughing, one scavenging and one fine selection is conducted by adopting amixed collecting agent which is prepared from an anion collecting agent sodium oleate and oleamide at a proportion. The method is low in cost and easy and convenient to operate, a calcium component cover is promoted to be formed on the surface of pyrites through sodium carbonate and calcium hypochlorite, the calcium activated particle density on the surface of the phosphorus-containing ore is increased, and synchronous removal of phosphorus and sulfur and improvement of iron grade are achieved through the mixed collecting agent with good selectivity.
Owner:KUNMING UNIV OF SCI & TECH
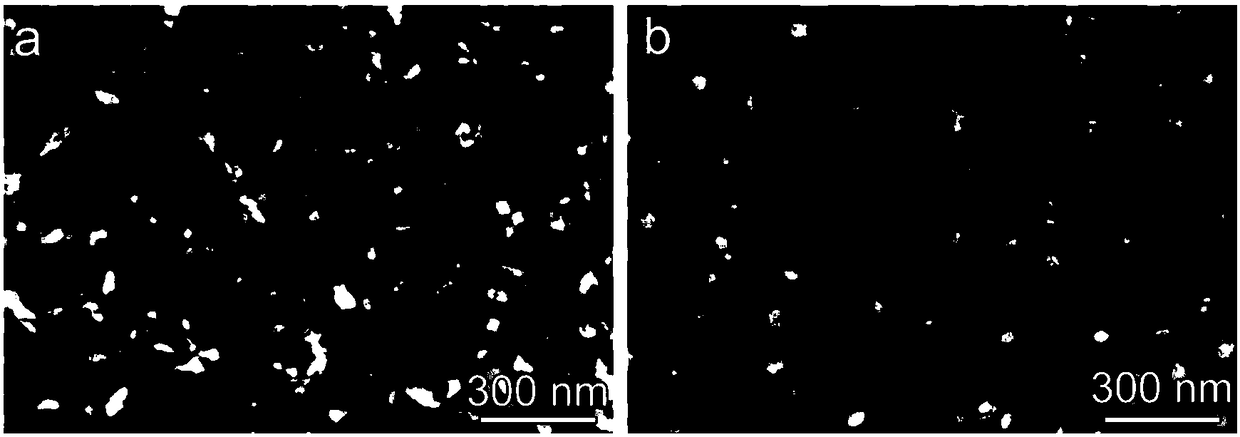
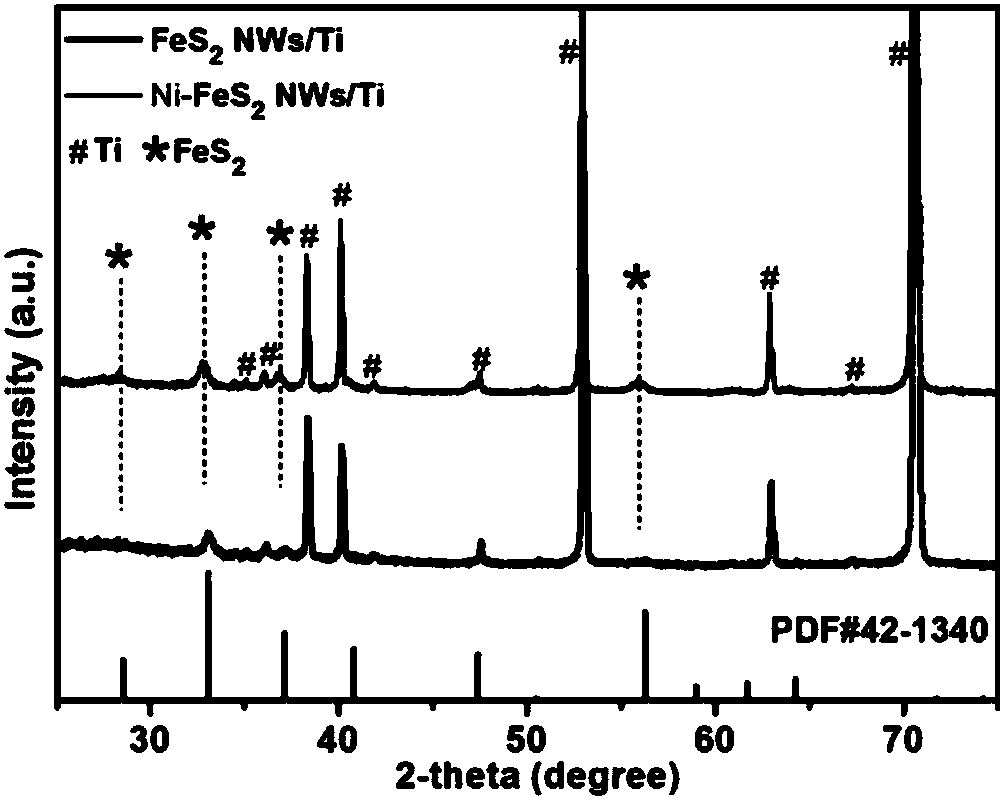
Synthesis method of nickel-doped iron disulfide nanowire array structure on titanium sheet substrate
ActiveCN108321388AAvoid loss of electrical conductivityStable structureMaterial nanotechnologyPhysical/chemical process catalystsNickel saltElectrolysis
The invention relates to a synthesis method of nickel-doped iron disulfide nanowire array structure on a titanium sheet substrate. The synthesis method comprises the following steps: preparing a mixedaqueous solution containing iron salt, nickel salt, sodium sulfate and urea, feeding a clean titanium sheet, and performing hydrothermal reaction to obtain a nickel-doped iron oxide hydroxide nanowire array growing on the surface of the titanium sheet substrate in situ; placing a precursor into a tubular furnace for high-temperature gas phase vulcanization, and performing atmosphere protection byusing argon, thus obtaining the nickel-doped iron disulfide nanowire array assembled on the titanium sheet substrate. The method is easy and convenient to operate and high in repeatability; an obtained product is stable in structure, can be uniformly and firmly distributed on the surface of the titanium sheet, and can be directly used as a two-dimensional electrode material applied into electrochemical equipment; in addition, electrolytic water tests show that doping of nickel substantially improves the electrocatalytic hydrogen production activity and the stability of iron disulfide and is expected to further promote improvement of the performance of the nickel-doped iron disulfide nanowire array structure in the field such as energy storage and photocatalysis, and the application rangeof the nickel-doped iron disulfide nanowire array structure is expanded.
Owner:TONGJI UNIV
Selective recovery of minerals by flotation
A method of recovering a target mineral from an ore containing the target mineral and an iron sulphide mineral comprising the steps of: a) grinding the ore to liberate target mineral from the iron sulphide mineral; b) forming a pulp of said ore; c) selecting a collector having the structure as follows: X-R-Y where R is a branched or straight chain hydrophobic hydrocarbon or polyether chain, and X and Y represent metal coordinating functional groups, d) add the collector to the pulp at a concentration at which the target mineral is able to be floated in preference to the iron sulphide mineral; and e) subjecting the pulp to froth flotation. The metal coordinating sulphur based functional groups may be identical or different.
Owner:COMMONWEALTH SCI & IND RES ORG
Preparation method of defective cobalt-doped iron disulfide porous hollow flower-like nano-powder and electrocatalytic application
The invention provides a preparation method of defective cobalt-doped iron disulfide porous hollow flower-like nano-powder and electrocatalytic application. The preparation method comprises the following steps: preparing an iron and cobalt reaction solution, and heating to synthesize amorphous cobalt ferrite nano-powder; carrying out a vulcanization reaction by a solvothermal method so as to prepare cobalt-doped iron disulfide flower-like nano-powder; and finally, annealing in inert gas shielding, thereby obtaining the defective cobalt-doped iron disulfide porous hollow flower-like nano-powder. The defective cobalt-doped iron disulfide porous hollow flower-like nano-powder has excellent catalytic performance when applied to an electrocatalytic oxygen production reaction (OER), the overpotential is low to 0.270V (relative to a standard hydrogen electrode), and the Tafel slope is low to 40 mV / dec.
Owner:UNIV OF JINAN
Marmatite flotation activating agent and preparation method and application thereof
The invention relates to a marmatite flotation activating agent, a preparation method and application thereof, belonging to the field of mineral processing flotation reagents. The invention aims at solving the technical problems that the activation selectivity of the marmatite flotation activating agent is weak, thereby leading the existence of lots of minerals such as ferric sulfide in marmatiteand the low recovery rate of the marmatite. According to the technical scheme, the marmatite flotation activating agent is prepared by reaction of 52-56% of copper sulfate, 12-15% of lead nitrate, 10-13% of sodium sulfide, 8-11% of oxidized paraffin soap and 9-12% of lauric acid under high temperature and high pressure. The marmatite flotation activating agent has good stability and strong selectivity. Marmatite concentrate with the grade of 46.53%-49.54% and the recovery rate of 78%-83.34% is obtained by a three-rough one-sweeping two-refining floatation technology under the combined action of the marmatite flotation activating agent and other flotation reagents such as lime, sodium n-butylxanthate and oil NO.2 in the marmatite flotation process.
Owner:四川省有色矿冶科技有限公司
Mineral processing technology of recycling tin metal from high sulphide ore
InactiveCN104722392ASlightly affectedImprove protectionFlotationWet separationIron(III) sulfideFoaming agent
The invention relates to a mineral processing technology for recycling tin metal from high sulphide ore. The mineral processing technology for recycling the tin metal from the high sulphide ore is characterized in that desulfuration floatation is firstly conducted on tinstone polymetallic sulphide ore, tin valuable mineral is recycled from the sulphide ore, and sulphide ore collector agents and foaming agents are applied to floatation. Preferably, ferric sulfide in the sulphide ore are separated and floated through floatation, enrichment of valuable mineral entering reselection is improved, and separation is conducted on valuable tin-containing mineral. Preferably, floatation equipment comprises a roughing floatation machine, a scavenging floatation machine and a concentration floatation machine; reselection equipment comprises a roughing shaking table and a scavenging shaking table. Preferably, a settling pond or a concentration pond is arranged at the front end of a mill. Preferably, sulphide ore pulp with the concentration ranging from nine percent to twenty percent is sedimented and concentrated to the sulphide ore with the concentration ranging from sixty percent to eighty percent through the concentration pond. Preferably, the sulphide ore collector agents are butyal xanthate, and the foaming agents is 2# oil. The mineral processing technology for recycling the tin metal from the high sulphide ore can improve the recycle utilization rate of the tin metal, and the environmental friendliness is high.
Owner:JIANGXI RARE EARTH & RARE METALS TUNGSTEN GRP HLDG CO LTD
Preparation method of small-size iron disulfide nano hollow spheres
ActiveCN110627132AGood size controlEasy to control crystallinityCell electrodesSecondary cellsDispersityIron(III) sulfide
The invention discloses a preparation method of small-size iron disulfide nano hollow spheres, and aims to solve the problem that a small-size hollow structure is difficult to obtain by an existing FeS2 powder preparation process. The method comprises the following steps: 1, preparing an iron stearate solution; 2, preparing a sulfur powder solution; 3, preparing a surfactant solution; and 4, respectively heating and stirring the iron stearate solution, the sulfur powder solution and the surfactant solution for reaction, then mixing, finally carrying out hydrothermal reaction, centrifuging a hydrothermal reaction product, and cleaning a solid obtained by centrifugation to obtain the small-size iron disulfide hollow nanospheres. The size of the prepared hollow FeS2 ranges from 10nm to 200nm,and the hollow FeS2 has good crystallinity and dispersity. The method is suitable for preparing the small-size iron disulfide hollow nanospheres.
Owner:HEILONGJIANG INST OF TECH
Organic electroluminescent device and preparation method thereof
InactiveCN104659250AImprove conductivityIncrease transfer rateSolid-state devicesSemiconductor/solid-state device manufacturingIron(III) sulfidePhenyl group
An organic electroluminescent device comprises a positive pole, a hole injection layer, a hole transporting layer, a luminescent layer, an electron transfer layer, an electron injection layer and a negative pole which are sequentially laminated, wherein the electron injection layer consists of a cesium salt doped layer and a ferric salt layer; the cesium salt doped layer comprises a bipolar organic transmission material and a cesium salt; the bipolar organic transmission material is chosen from at least one of 2,4,6-tri(N-phenyl-1-naphthylamine)-1,3,5-triazine, 2,6-bi(3-(9H-carbazole-9-yl)phenyl) pyridine, 3'3''-(4-(naphthalene-1-yl)-4H-1,2,4-triazole-3,5-biyl)bi(N,N-bi(xenyl)-4-ammonia) and 2,5-bi(4-(9-(2-ethylhexyl)-9H-carbazole-3-yl)phenyl)-1,3,4-oxadiazole, and the ferric salt layer is made from at least one of ferric chloride, ferric bromide and ferric sulfide.
Owner:OCEANS KING LIGHTING SCI&TECH CO LTD +2
Golden yellow crystal bead embryo and preparation method thereof
The invention discloses a golden yellow crystal bead embryo and a preparation method of the golden yellow crystal bead embryo. The golden yellow crystal bead embryo is prepared from the following components in parts by weight: 150-220 parts of quartz sands, 75-95 parts of pure alkali, 21-32 parts of potassium carbonate, 1-3 parts of boric acid, 2-5 parts of aluminum hydroxide, 1-3.5 parts of salts, 0.3-0.6 part of flour, 0.15-0.3 part of sulphur and 0.001-0.003 part of ferric sulfide. When the golden yellow crystal bead embryo is prepared, redox equilibrium of copper ions in a melting process is kept stable, so that a good dyeing property is realized; by using lead-free raw materials, the golden yellow crystal bead embryo is high in glossiness, bright in colors, order, high in yield and free of lead, and color difference, flower patterns and pollution are avoided; moreover, the production cost is greatly reduced, and a good production benefit is achieved.
Owner:浙江鑫伟兴水晶有限公司
Anode material of lithium-iron disulfide battery
ActiveCN106898771AIncrease contentLarge specific surface areaCell electrodesLi-accumulatorsIron(III) sulfideCopper oxide
The invention discloses an anode material of a lithium-iron disulfide battery. The anode material comprises a modified iron disulfide anode active material which is iron disulfide doped with, by mass, 2% of titanium oxide, 4% of magnesium oxide and 6% of copper oxide. A preparation method of the anode active material specifically includes a) preparing first heat-treated iron disulfide, second heat-treated iron disulfide and third heat-treated iron disulfide; b) preparing first cladding iron disulfide, second cladding iron disulfide and third cladding iron disulfide; c) uniformly mixing the first cladding iron disulfide, the second cladding iron disulfide and the third cladding iron disulfide so as to obtain the anode material of the lithium-iron disulfide battery. The anode material of the lithium-iron disulfide battery has the advantages that open-circuit voltage of the battery can be reduced, so that a voltage platform is steadier; a composite electrically-conductive agent is added into the anode material, and accordingly electric conductivity can be improved greatly, battery polarization is reduced, high-and-low-temperature performance of the battery is enhanced and service life of the battery is prolonged.
Owner:LIUZHOU HAOXIANGTE SCI & TECH
Organic electroluminescence device and preparation method thereof
InactiveCN104659246AImprove conductivityImprove light extraction efficiencySolid-state devicesSemiconductor/solid-state device manufacturingIron(III) sulfideRubidium compound
An organic electroluminescence device comprises an anode, a hole injection layer, a hole transporting layer, a luminous layer, an electron transporting layer, an electron injection layer and a cathode which are overlapped in sequence, wherein the electron injection layer comprises a rubidium compound doped layer and a metal doped layer; the rubidium compound doped layer is made of a rubidium compound material and a molysite material doped in the rubidium compound material; the rubidium compound material is one or more of rubidium carbonate, rubidium chloride, rubidium nitrate and rubidium sulfate; the molysite material is one or more of ferric chloride, ferric bromide and ferric sulfide; the metal doped layer is made of a first metal material and a second metal material doped in the first metal material; the work function of the first metal material is -2.0 eV to -3.5 eV; the work function of the second metal material is -4.0 eV to -5.5 eV. The light efficiency of the organic electroluminescence device is relatively high. The invention further provides a preparation method of the organic electroluminescence device.
Owner:OCEANS KING LIGHTING SCI&TECH CO LTD +2
Organic electroluminescence device and preparation method thereof
InactiveCN104659234ASolution to short lifeLarge specific surface areaSolid-state devicesSemiconductor/solid-state device manufacturingIron(III) sulfideMagnesium sulfide
The invention discloses an organic electroluminescence device which comprises a glass substrate, a scattering layer, an anode, a hole injection layer, a hole transmission layer, a light emitting layer, an electron transfer layer, an electron injection layer and a cathode which are overlapped in sequence, wherein the scattering layer consists of a ternary doped layer and a ferric salt doped layer; the ternary doped layer comprises titanium dioxide, a magnesium compound material and a light emitting material; the magnesium compound material is selected from at least one of magnesium fluoride, magnesium oxide and magnesium sulfide; the ferric salt doped layer comprises a ferric salt material and an organic silicon micromolecule material doped in the ferric salt material; the ferric salt material is selected from at least one of ferric chloride, ferric bromide and ferric sulfide; the energy gap of the organic silicon micromolecule material is minus 3.5-minus 5.5eV. The organic electroluminescence device disclosed by the invention is relatively high in light emission efficiency. The invention further provides a preparation method of the organic electroluminescence device.
Owner:OCEANS KING LIGHTING SCI&TECH CO LTD +2
Organic electroluminescent device and preparation method thereof
InactiveCN104659214AIncrease transfer rateImprove injection abilitySolid-state devicesSemiconductor/solid-state device manufacturingLight-emitting diodePyran
An organic electroluminescent device comprises an anode, a hole injection layer, a hole transport layer, a light emitting layer, an electron transport layer, an electron injection layer, and a cathode, which are stacked in sequence. The electron injection layer is composed of a first ferric salt layer, a ternary doped layer, and a second ferric salt layer. The first ferric salt layer and the second ferric salt layer are made of at least one material selected from ferric chloride, ferric bromide and ferric sulfide. The ternary doped layer is composed of a bipolar metal oxide material, a luminescent material and zinc powder. The bipolar metal oxide material includes at least one selected from molybdenum trioxide, tungsten trioxide and vanadium pentoxide. The luminescent material includes at least one selected from 4-(dinitrile methyl)-2-butyl-6-(1,1,7,7-tetramethyljulolidine-9-vinyl)-4H-pyrans, 9,10-di-beta-naphthylene anthracene, 4,4'-bis(9-ethyl-3-carbazole vinyl)-1,1'-biphenyl and 8-hydroxyquinoline aluminum.
Owner:OCEANS KING LIGHTING SCI&TECH CO LTD +2
Production method of crude lead smelting environment-friendly copper removing agent
ActiveCN109046713ANo pollution in the processEmission reductionGrain treatmentsWet separationEnvironmental resistanceLead smelting
The invention relates to a production method of a crude lead smelting environment-friendly copper removing agent. According to the technical scheme, the production method comprises the steps that ferric sulfide is crushed multiple times, impurities such as sand in ferric sulfide particles are removed through a jigging machine, and drying is conducted after stirring to remove water. The sulphur content of the produced copper removing agent is higher than 52%, no pollution or smoke is generated, emission of sulfur dioxide is reduced, no irritant gas is generated, no low-level pollution is generated, and the copper content in lead is lower than 0.00015% after copper removing. Environment-friendly devices such as a biomass hot blast furnace, a bag-type dust collector and a cyclone dust collector are widely adopted in the production process, green production is achieved, and pollution to the environment in the production process is reduced.
Owner:栾川县亨凯冶金材料销售有限公司
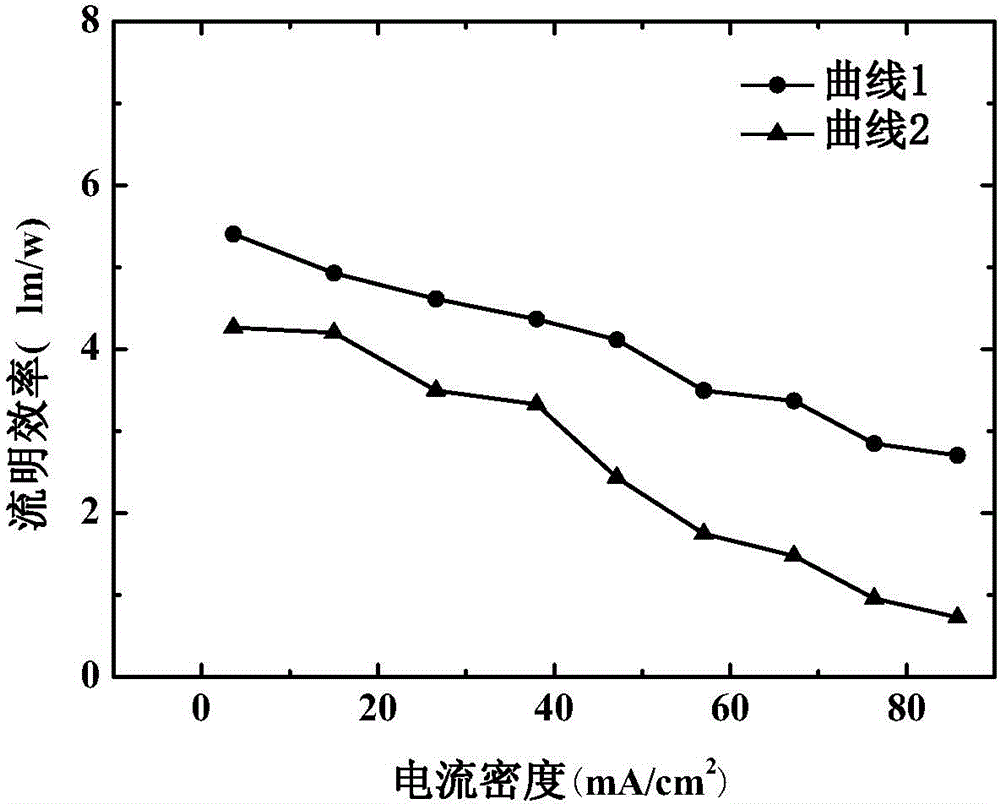
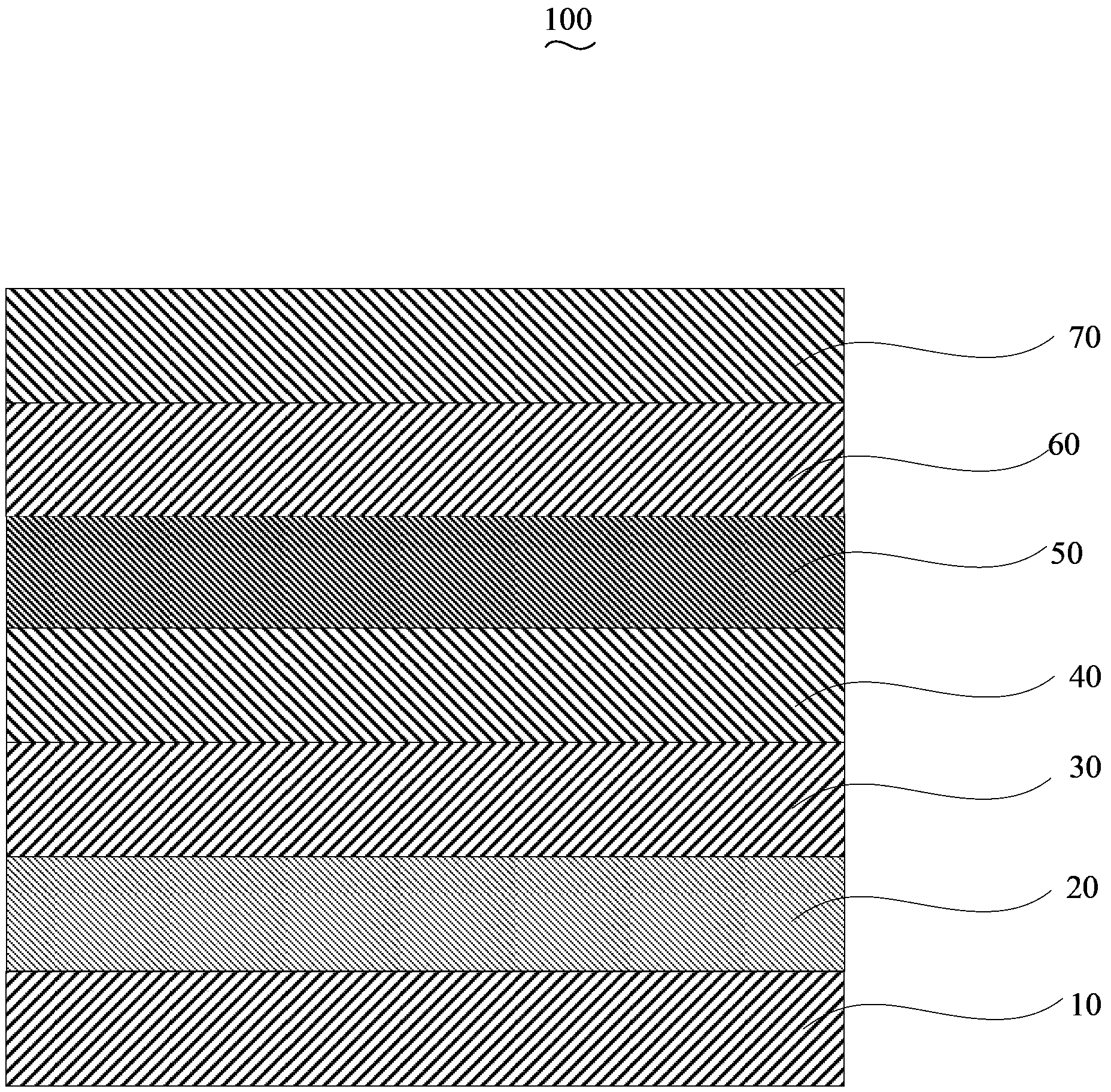
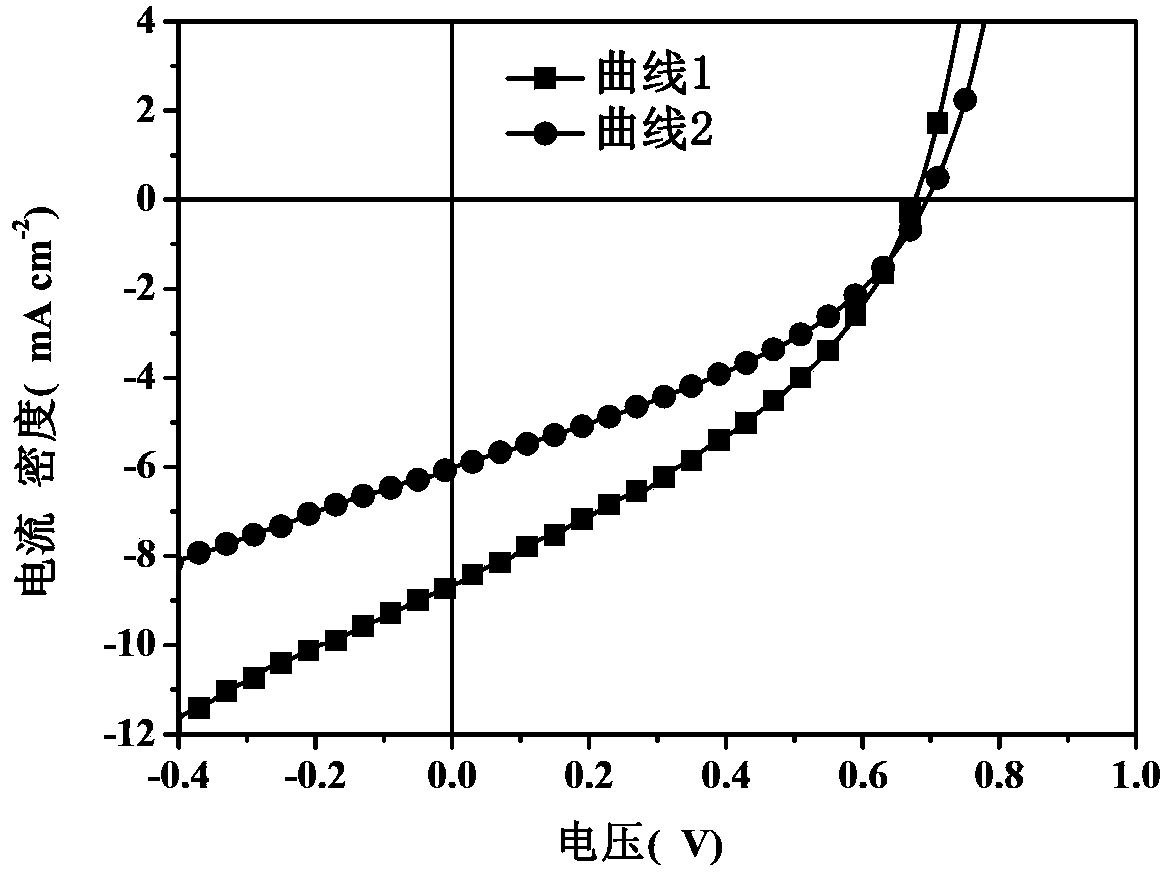
Solar cell device and method for manufacturing same
InactiveCN104253212AImprove light absorption efficiencyAvoid problemsFinal product manufactureSolid-state devicesIron(III) sulfideHigh energy
The invention discloses a solar cell device. The solar cell device comprises an anode, a hole buffer layer, a first active layer, an intermediate layer, a second active layer, an electron buffer layer and a cathode which are sequentially stacked on one another. The first active layer and the second active layer are made of mixtures of poly-3-hexyl-thiophene and 6,6-phenyl-C<61>-methyl butyrate, the intermediate layer is made of ferric salt, polythiophene and electron transport materials, the selected ferric salt comprises at least one type of ferric chloride, ferric tribromide and ferric sulfide, the elected polythiophene comprises at least one type of poly-3-hexyl-thiophene, poly-3-methyl-thiophene and poly-3-octyl-thiophene, and the selected electron transport materials comprise at least one type of 4,7-diphenyl-1,10-phenanthroline, 3-(biphenyl-4-based)-5-(4-tertiary butyl phenyl)-4-phenyl-4H-1,2,4-triazole and N-aryl benzimidazole. The solar cell device has the advantage of high energy conversion efficiency. The invention further provides a method for manufacturing the solar cell device.
Owner:OCEANS KING LIGHTING SCI&TECH CO LTD +2
Organic electroluminescent device and preparation method thereof
InactiveCN104659243ALower injection barrierImprove injection efficiencySolid-state devicesSemiconductor/solid-state device manufacturingIron(III) sulfideRubidium compound
An organic electroluminescent device comprises an anode, a hole injection layer, a hole transport layer, a light emitting layer, an electron transport layer, an electron injection layer, and a cathode, which are stacked in sequence. The electron injection layer is composed of a rubidium compound doped layer and a ferric salt doped layer. The rubidium compound doped layer includes a rubidium compound material group and an electron transport material doped in the rubidium compound material. The rubidium compound material includes at least one selected from rubidium carbonate, rubidium chloride, rubidium nitrate, and rubidium sulfate. The electron transport material includes at least one selected from 4,7-diphenyl-1,10-phenanthroline, 2-(4'-tert-butylphenyl)-5-(4'-biphenyl)-1,3,4-oxadiazole, 8-hydroxyquinoline aluminum, and N-arylbenzimidazole. The ferric salt doped layer includes a ferric salt material and a bipolar organic transport material doped in the ferric salt material. The ferric salt material includes at least one selected from ferric chloride, ferric bromide and ferric sulfide.
Owner:OCEANS KING LIGHTING SCI&TECH CO LTD +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com