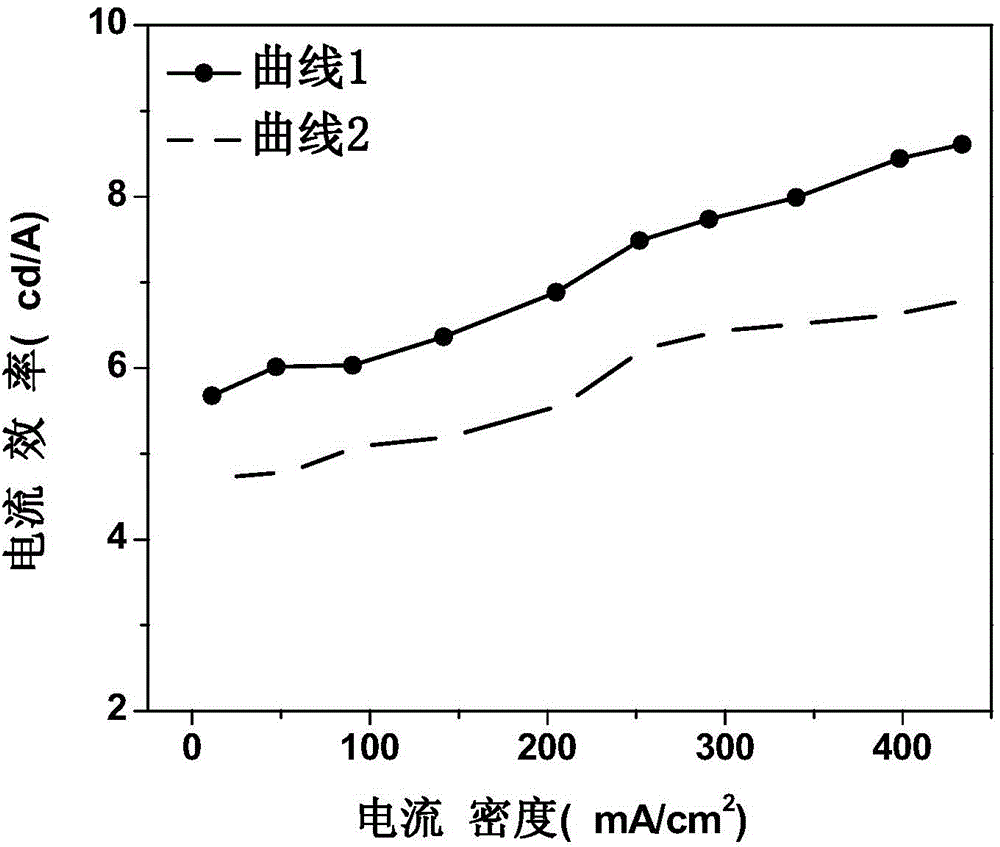
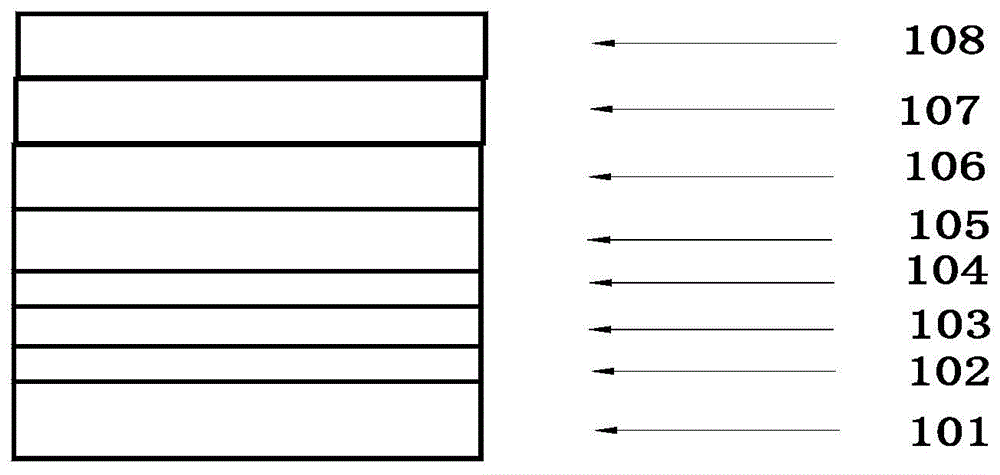
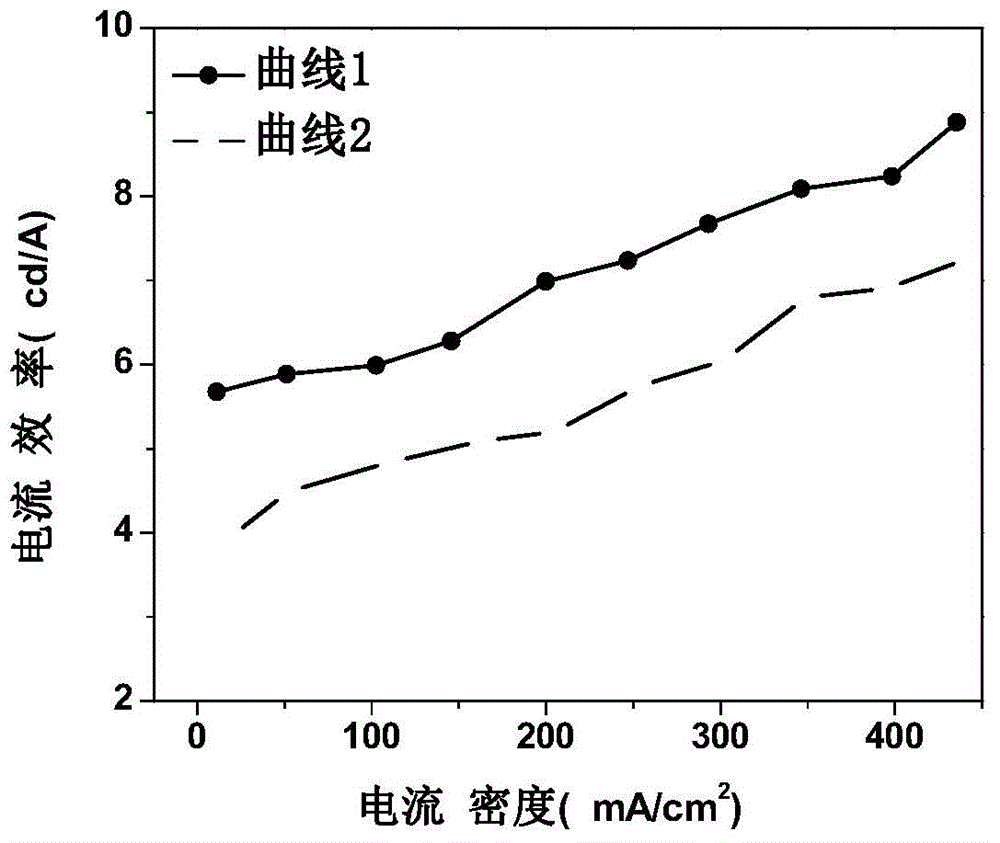
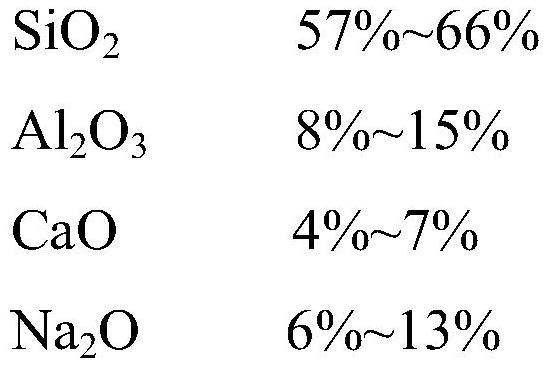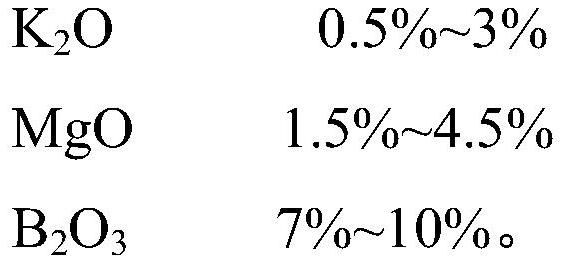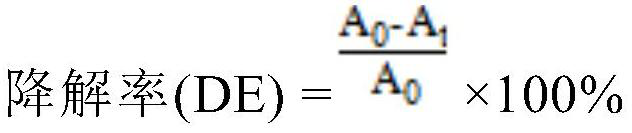Patents
Literature
40 results about "Rubidium nitrate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Rubidium nitrate is an inorganic compound with the formula RbNO₃. This alkali metal nitrate salt is white and highly soluble in water.
Method for preparing Nano tube of titanate
InactiveCN101003385ALarge specific surface areaImproved electrical and catalytic propertiesPolycrystalline material growthTitanium compoundsCopper nitrateSodium titanate
This invention discloses a method for preparing titanate nanotubes. The method comprises: uniformly mixing nitrate and power of sodium titanate nanotubes at a mol ratio of (5-100):1, performing melt-reaction at 50-450 deg.C for 3-48 h, cooling, washing to remove unreacted nitrate, and drying to obtain titanate nanotubes. The nitrate is lithium nitrate, and drying to obtain titanate nanotubes. The nitrate is lithium nitrate, potassium nitrate, rubidium nitrate, cesium nitrate, silver nitrate, nickel nitrate, thallium nitrate, copper nitrate, zinc nitrate or cobalt nitrate. The precursor of sodium titanate nanotubes is prepared by: reacting titanium dioxide, metatitanic acid or titanate ester in 20-80 wt. % NaOH aqueous solution at 100-140 deg.C for 12-72 h, washing with water, filtering and drying. This invention can prepare titanate nanotubes with large specific surface area by melt-exchange method.
Owner:HENAN UNIVERSITY
Cesium-rubidium-potassium monolithic flameproof glass and preparation method
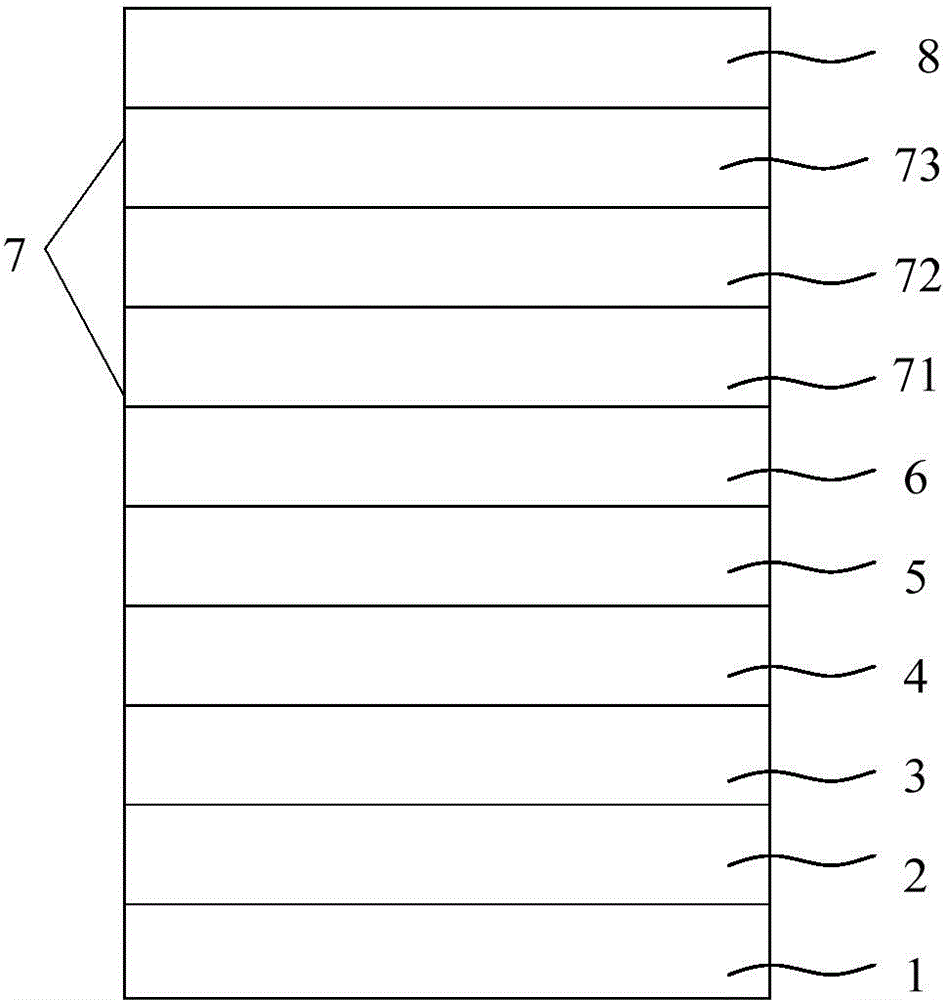
The invention discloses cesium-rubidium-potassium monolithic flameproof glass and a preparation method. The preparation method comprises a process of carrying out chemical strengthening on glass, wherein chemical strengthening is carried out by placing the glass in molten salts and carrying out ion exchange by a two-step method; in the first step, the molten salt is a mixture of potassium nitrate, rubidium nitrate, potassium fluoride and potassium hydroxide, the exchange temperature is 320-360 DEG C and the heat-insulation time is 2 hours; and in the second step, the molten salt is a mixture of potassium nitrate, cesium nitrate, aluminum oxide, boron fluoride, potassium carbonate and potassium phosphate, the exchange temperature is 500 DEG C and the heat-insulation time is 3 hours. The monolithic flameproof glass product prepared by the method is stable in quality and excellent in fire resistance; in the fire resistance test, the invalidation of the monolithic flameproof glass is caused by final softening and collapsing instead of heat bursting destruction; and the fire resistance time is 120 minutes.
Owner:SHENYANG JIANZHU UNIVERSITY
Castable infrared illuminant compositions
InactiveUS6190475B1Increase burn rateSmall and lightFirework flares/torchesExplosivesPolyesterPolyamide
Owner:NORTHROP GRUMMAN INNOVATION SYST INC
Castable infrared illuminant compositions
InactiveUS6123789AIncrease burn rateLittle visible lightFirework flares/torchesExplosivesPolyesterPolyamide
Compositions are provided which, when burned, produce significant levels of infrared radiation, but only limited levels of visible radiation. The basic components of the compositions include a binder, an oxidizer, and a fuel, where the binder also acts the fuel. Preferred oxidizers include those compounds which produce large quantities of infrared radiation when the flare composition is burned. Such oxidizers include potassium nitrate, cesium nitrate, rubidium nitrate, and combinations of these compounds. Selection of the binder is important in order to provide the composition with the desirable characteristics identified above. The binder of the present invention does not produce significant soot. At the same time, the binder serves to form a composition which is processible, avoids chunking, and is compatible with the oxidizers used. It has been found that polymer binders which include relatively short carbon chains (1-6 continuous carbon atoms) are preferred. Examples of such polymers include polyesters, polyethers, polyamides, and polyamines.
Owner:NORTHROP GRUMMAN INNOVATION SYST INC
Method of Producing a Partly or Completely Semi-Insulating or P-Type Doped ZnO Substrate, Substrates Obtained, and Electronic, Electro-Optic or Optoelectronic Devices Comprising Them
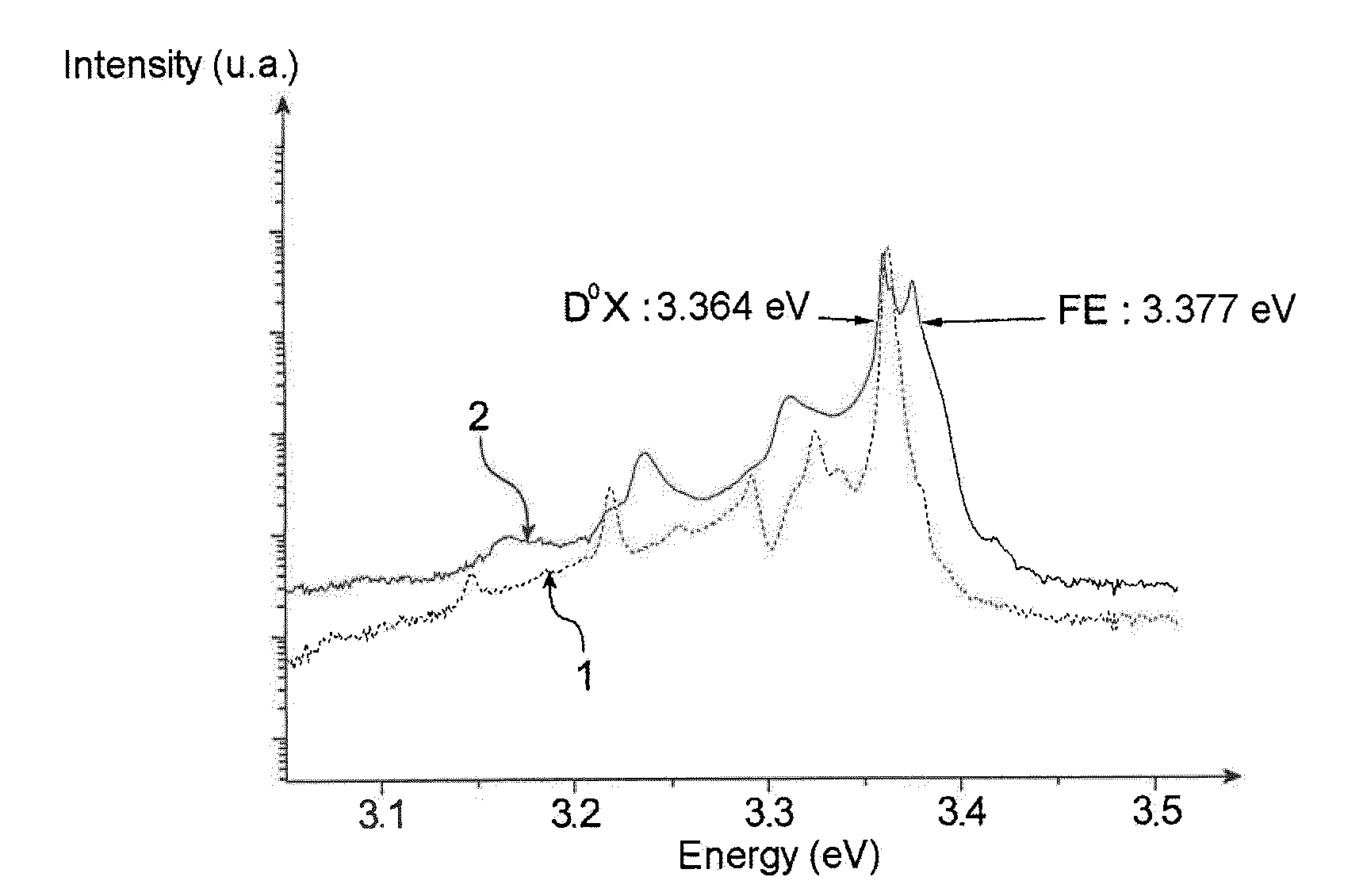
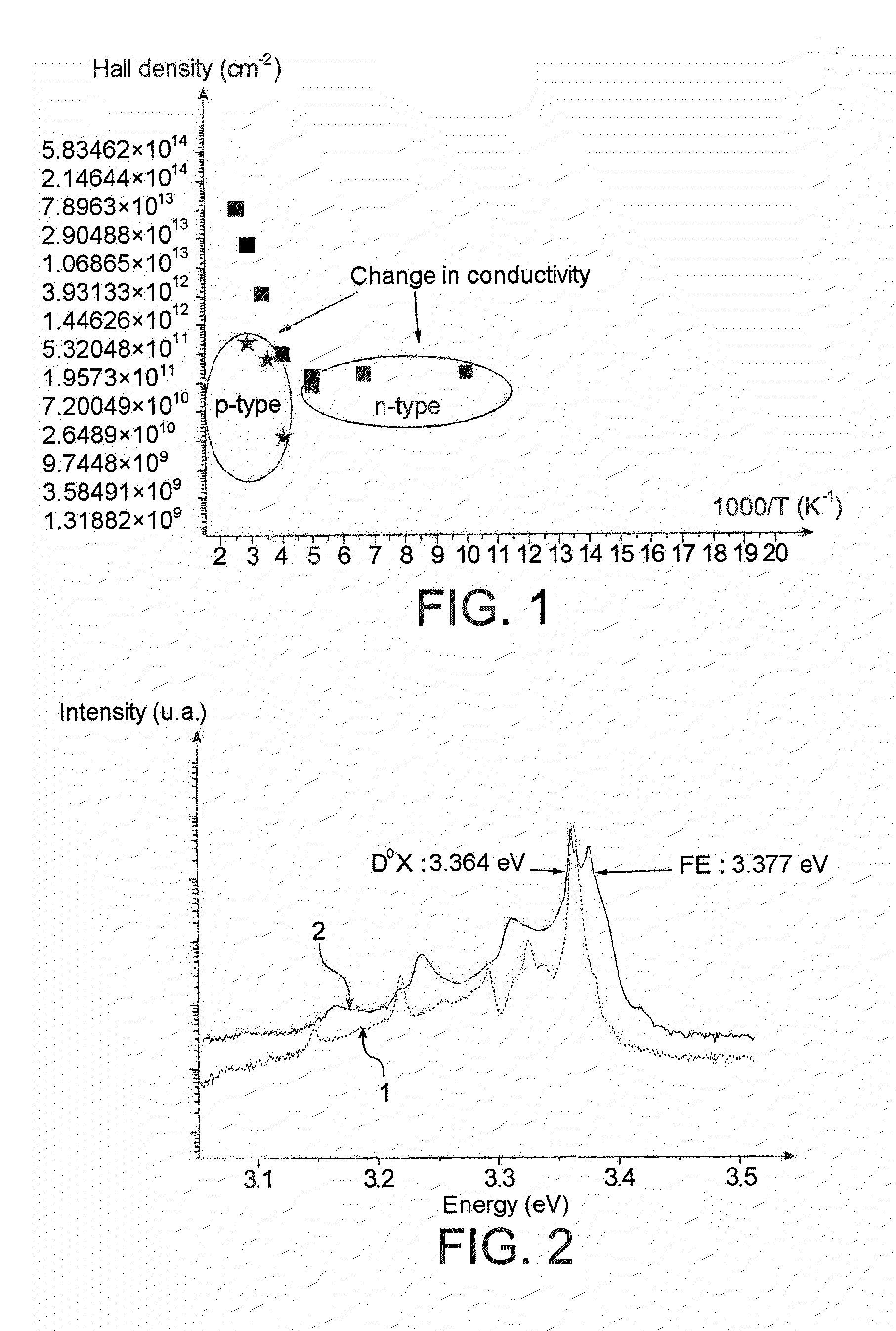
Method of producing a partly or completely semi-insulating or p-type doped ZnO substrate from an n-type doped ZnO substrate, in which the n-type doped ZnO substrate is brought into contact with an anhydrous molten salt chosen from anhydrous molten sodium nitrate, lithium nitrate, potassium nitrate and rubidium nitrate.Partly or completely semi-insulating or p-type doped ZnO substrate, said substrate being in particular in the form of a thin layer, film or in the form of nanowires ; and said substrate being doped at the same time by an element chosen from Na, Li, K and Rb; by N; and by O; it being furthermore possible for ZnO or GaN to be epitaxially grown on this substrate.Electronic, optoelectronic or electro-optic device such as a light-emitting diode (LED) comprising this substrate.
Owner:COMMISSARIAT A LENERGIE ATOMIQUE ET AUX ENERGIES ALTERNATIVES
Preparation method of environmentally-friendly formaldehyde degradation photocatalyst
InactiveCN110152740AWide variety of sourcesImprove stabilityOrganic-compounds/hydrides/coordination-complexes catalystsDispersed particle separationUltrasonic dispersionChemistry
The invention discloses a preparation method of an environmentally-friendly formaldehyde degradation photocatalyst. The method is characterized by comprising the following steps: preparing porous houttuynia cordata powder by adopting soluble starch, egg white and pretreated houttuynia cordata powder; preparing a rubidium-doped BiVO3 photocatalyst by adopting 4 mol / L nitric acid, vanadium pentoxide, rubidium nitrate and bismuth oxide; adding the following materials into a reactor according to percentages by mass: 54-58% of deionized water, 34-38% of the porous houttuynia cordata powder and 2-4%of polyacrylic acid, performing ultrasonic dispersion, adding 4-8% of the rubidium-doped BiVO3 photocatalyst, wherein a sum of percentages by mass of each component is 100%, continuing ultrasonic dispersion for 30 min, performing solid-liquid separation, performing washing by using deionized water, and performing drying to obtain the environmentally-friendly formaldehyde degradation photocatalyst. The environmentally-friendly formaldehyde degradation photocatalyst provided by the invention has the characteristics of a simple preparation method, good stability, degradability and environmentalfriendliness; and the photocatalyst has the characteristics of mild reaction conditions, high catalytic activity, a less usage amount and a high formaldehyde degradation rate.
Owner:UNIV OF JINAN
Ultrathin chemical toughened glass and preparation method thereof
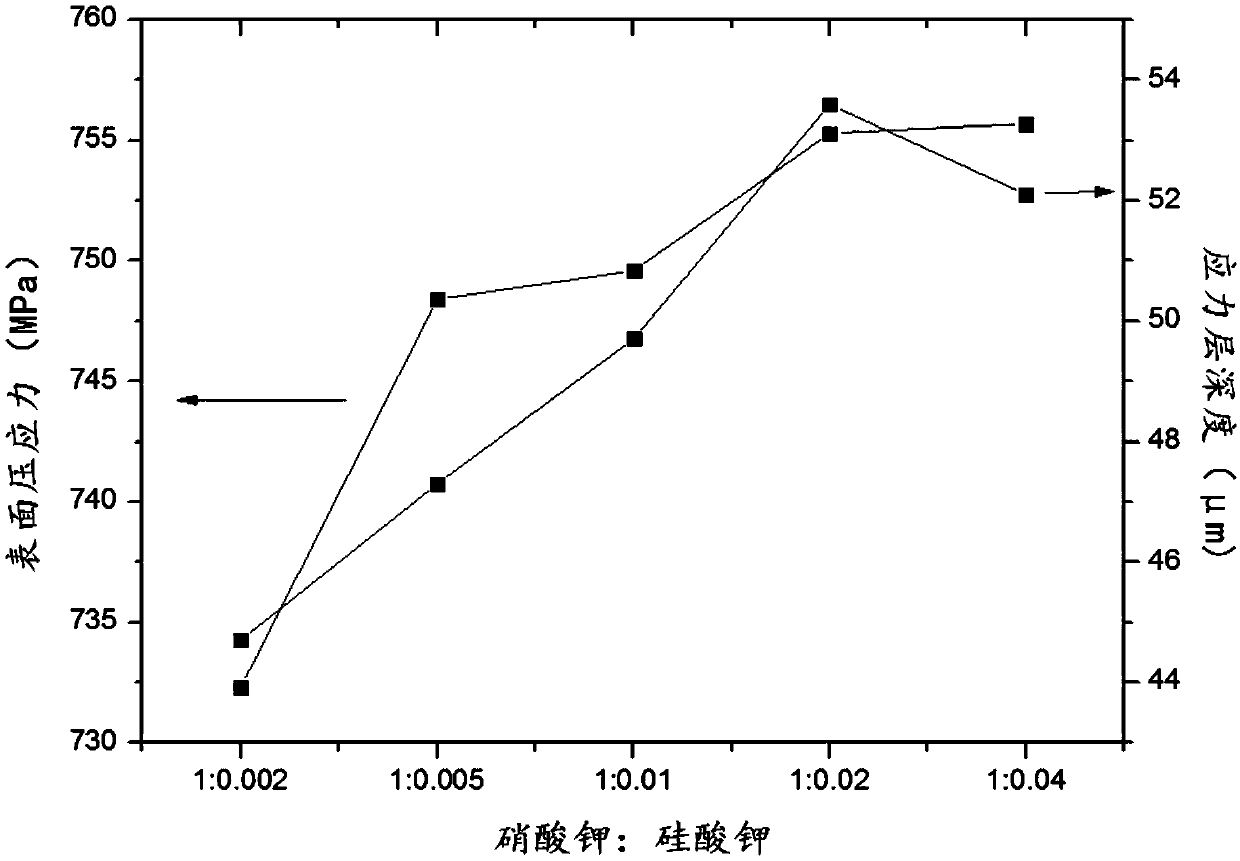
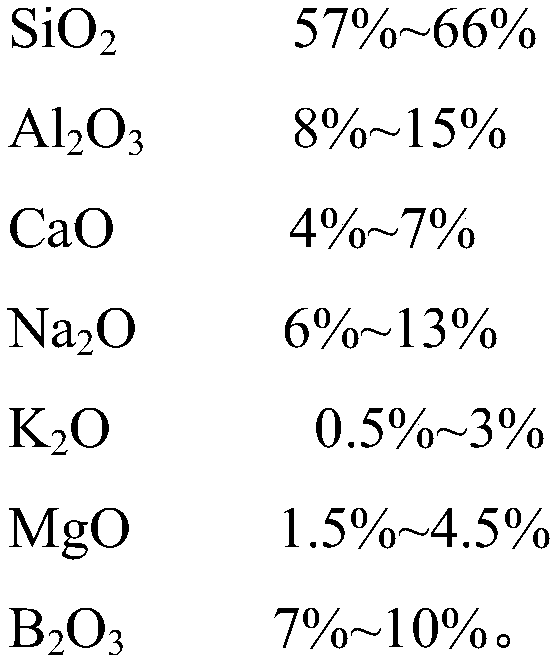
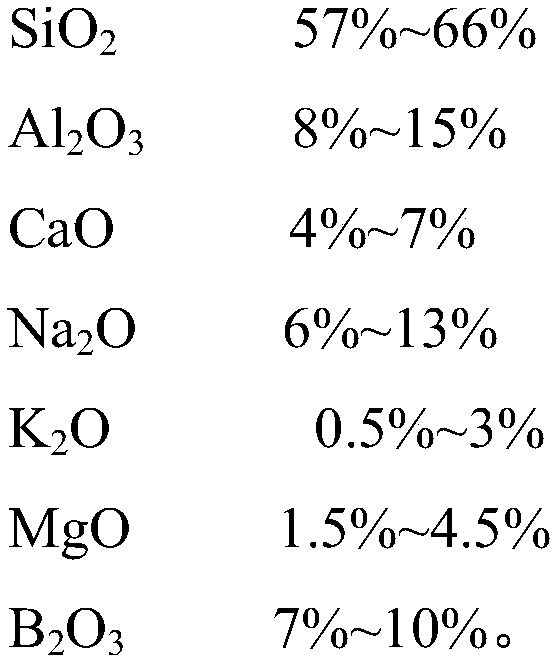
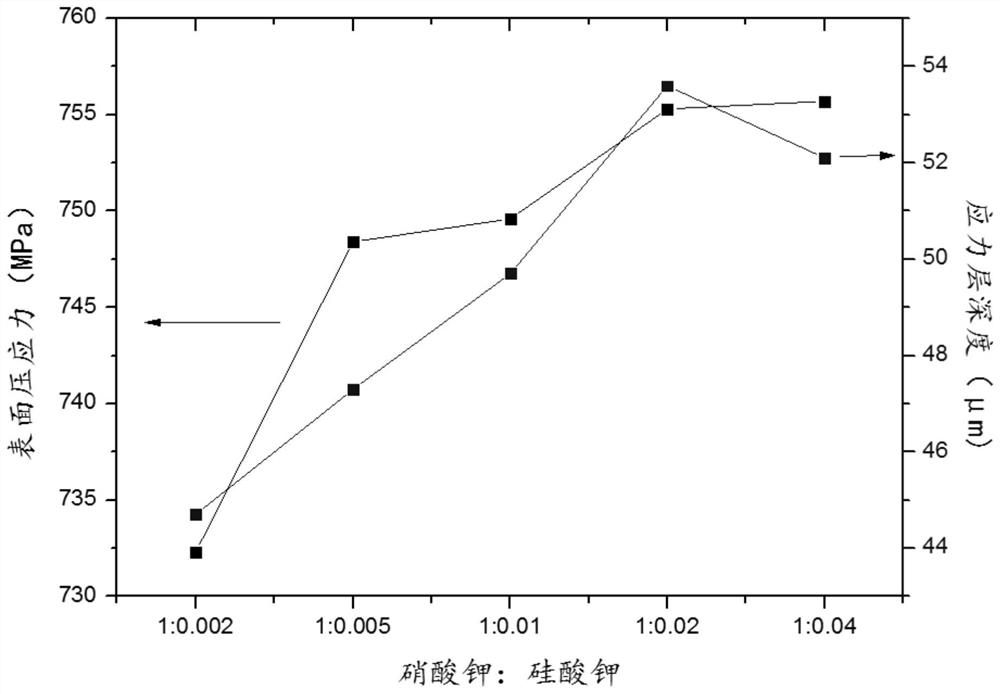
The invention discloses ultrathin chemical toughened glass and a preparation method thereof. The chemical toughened glass is obtained by immersing a glass sample into a microwave hydrothermal environment of an ion exchange solution for carrying out ion exchange treatment, wherein the ion exchange solution is a water solution prepared from potassium nitrate, potassium silicate, rubidium nitrate andcesium nitrate; in the ion exchange solution, the concentration of K<+> is 27 to 40 mol / L, the concentration of Rb<+> is 0.5 to 4 mol / L and the concentration of CS<+> is 1.5 to 4.5 mol / L; the mol ratio of the potassium nitrate to the potassium silicate is 1 to (0.002 to 0.05); the microwave hydrothermal temperature is 300 DEG C to 350 DEG C and the ion exchange time is 4 to 10 h. Compared with amolten-salt growth method, relatively less pollution and energy source wastes are generated; the stress depth of the prepared chemical toughened glass reaches 45 mum or more and the surface stress strength reaches 700 MPa or more; the chemical toughened glass has good light transparency.
Owner:WUJIANG GOLDEN GLASS TECH +1
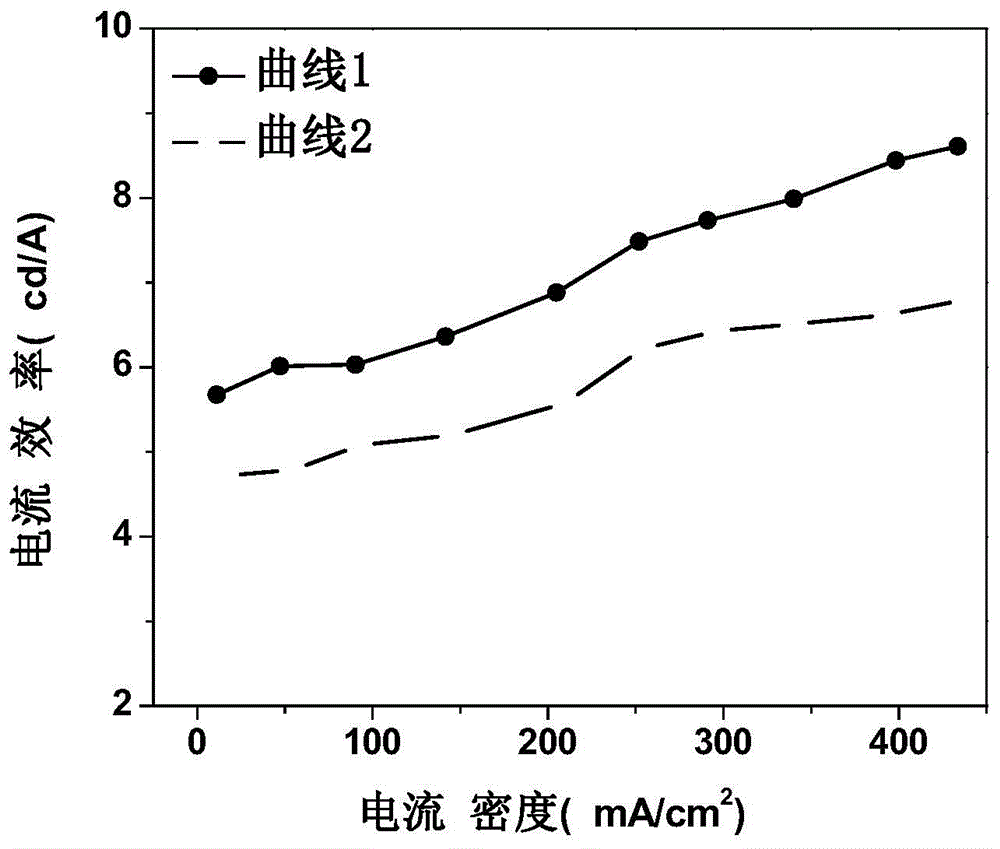
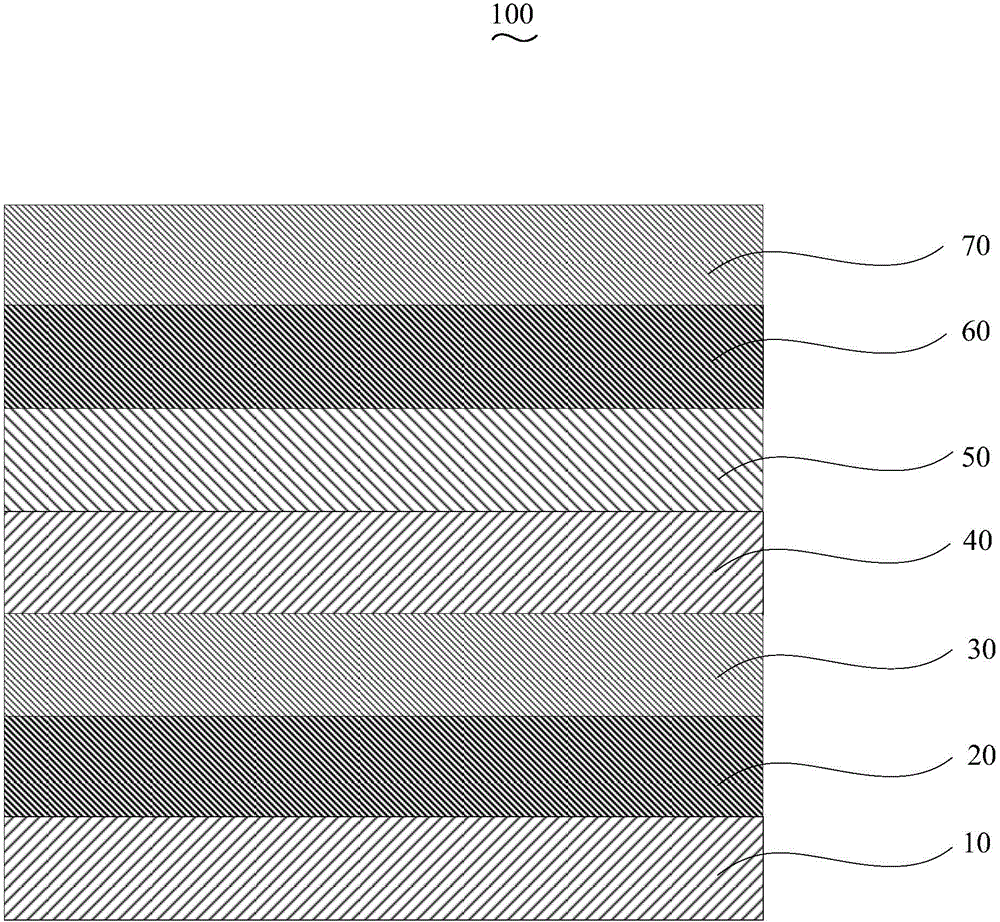
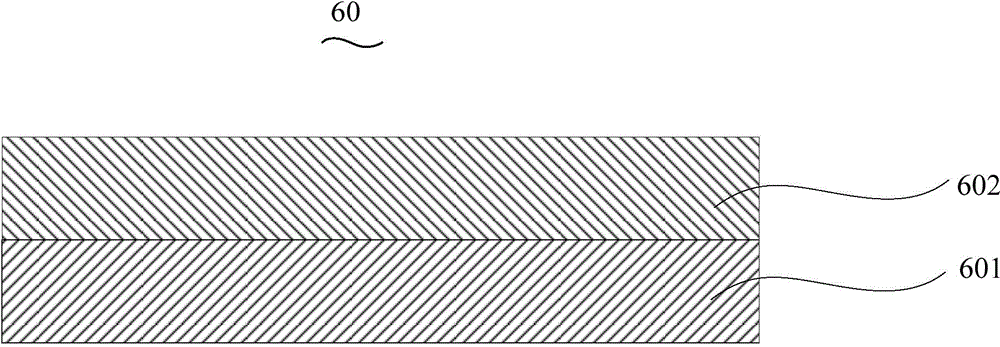
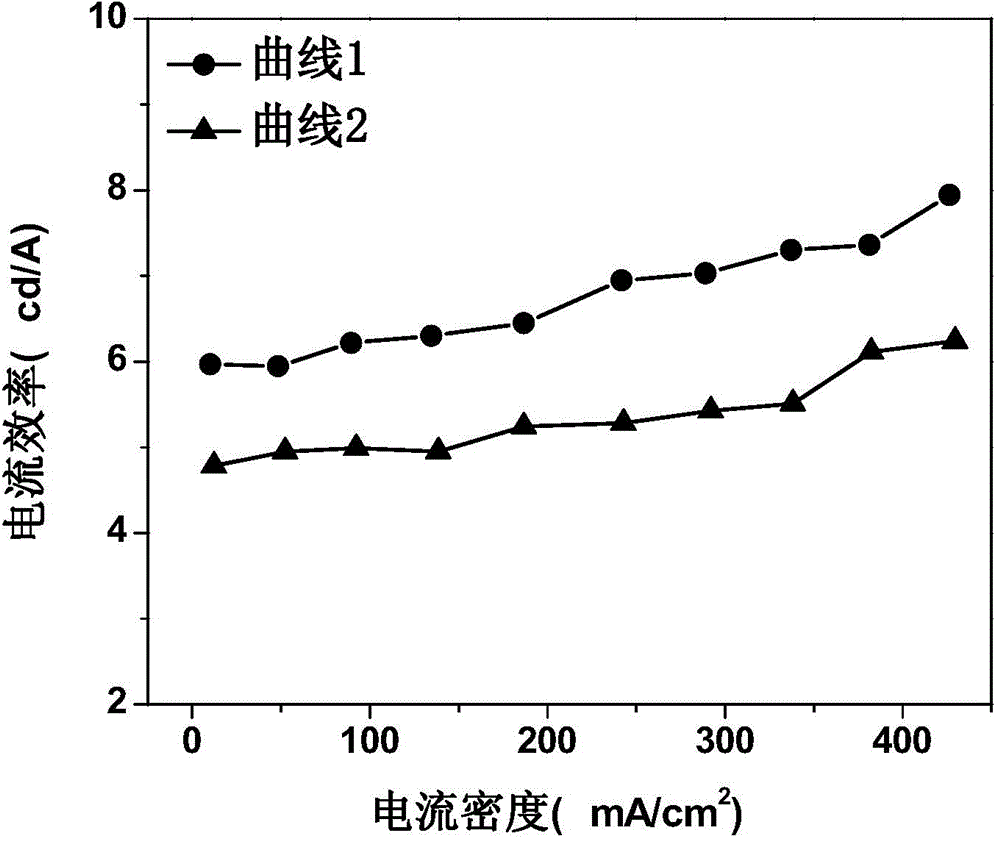
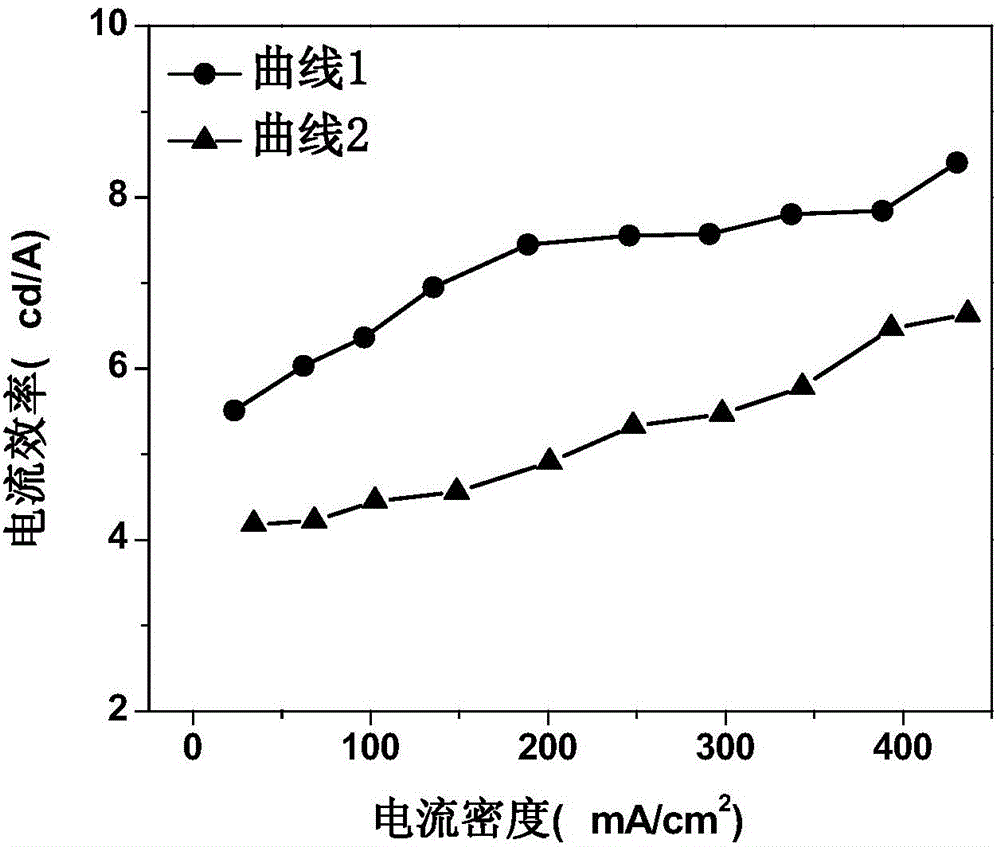
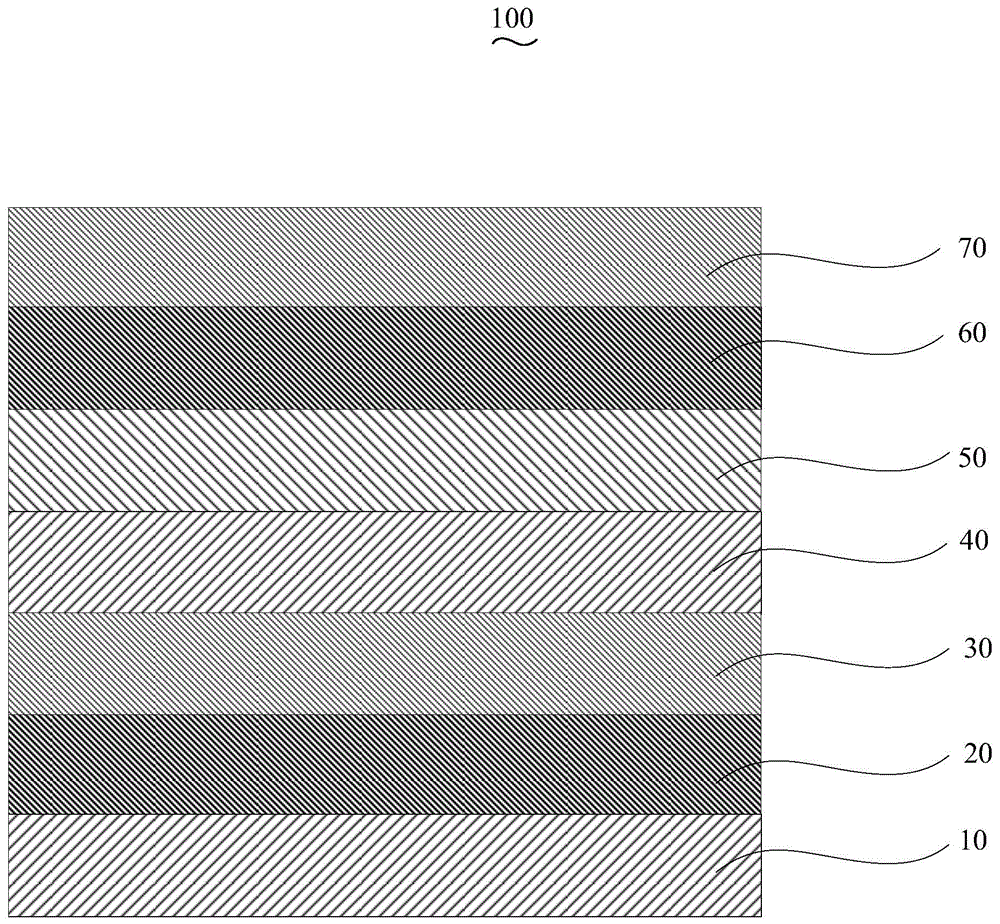
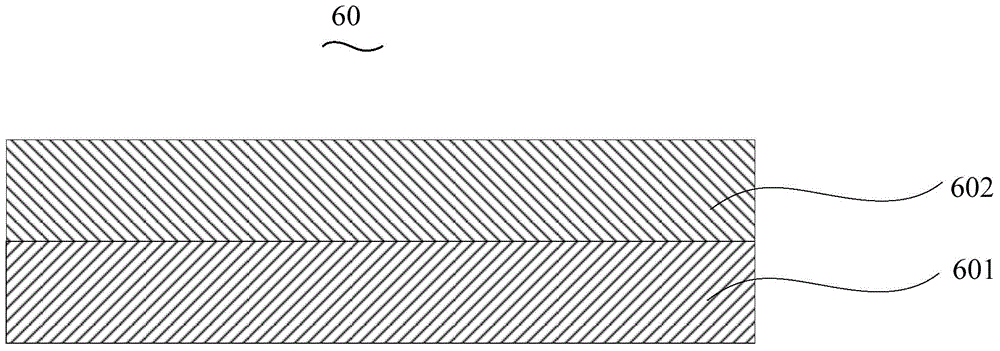
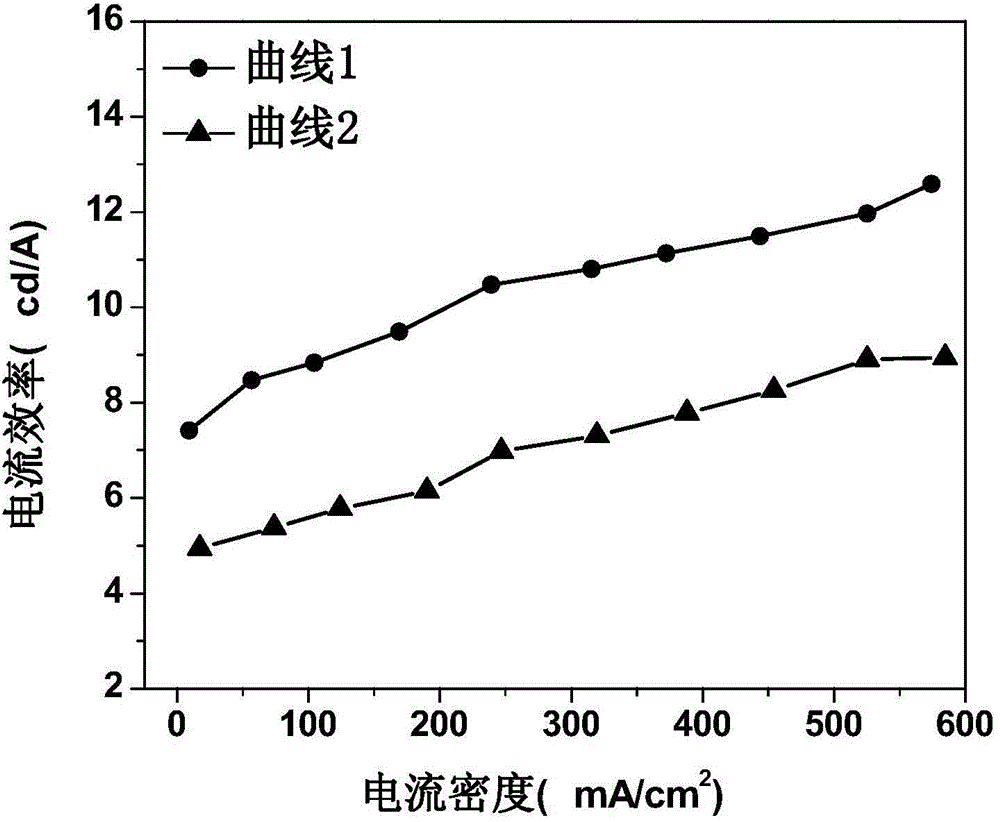
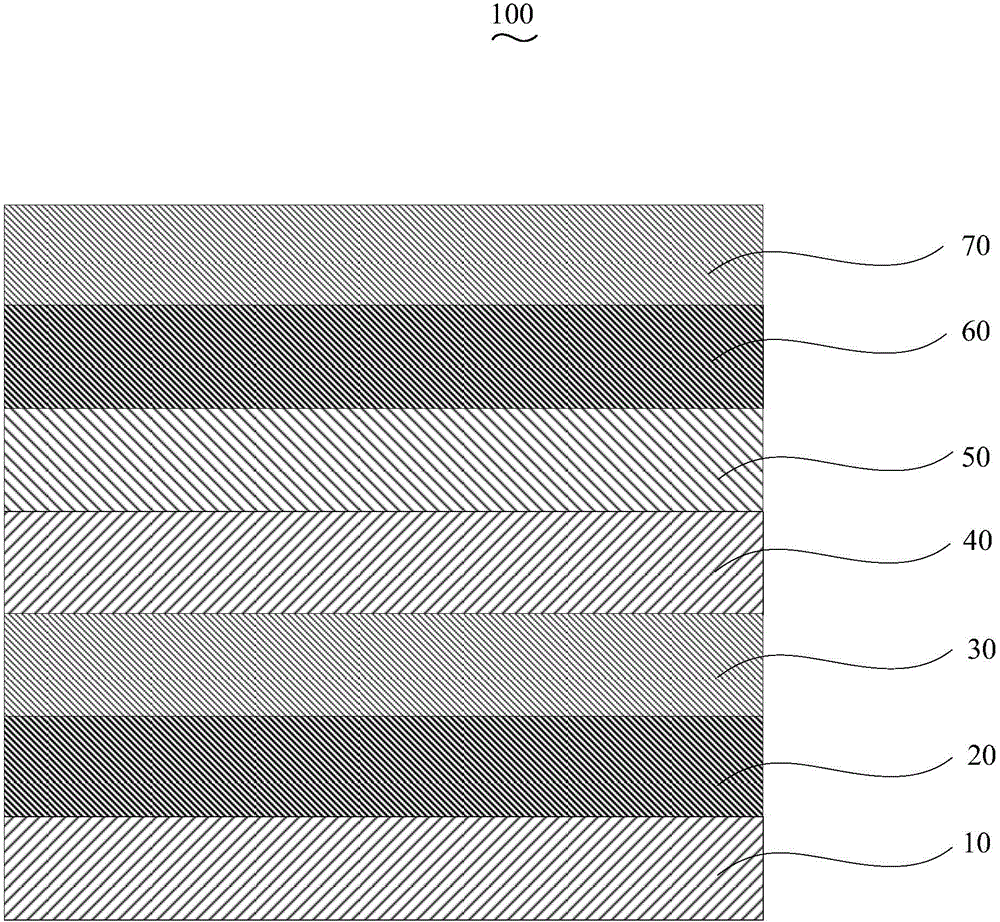
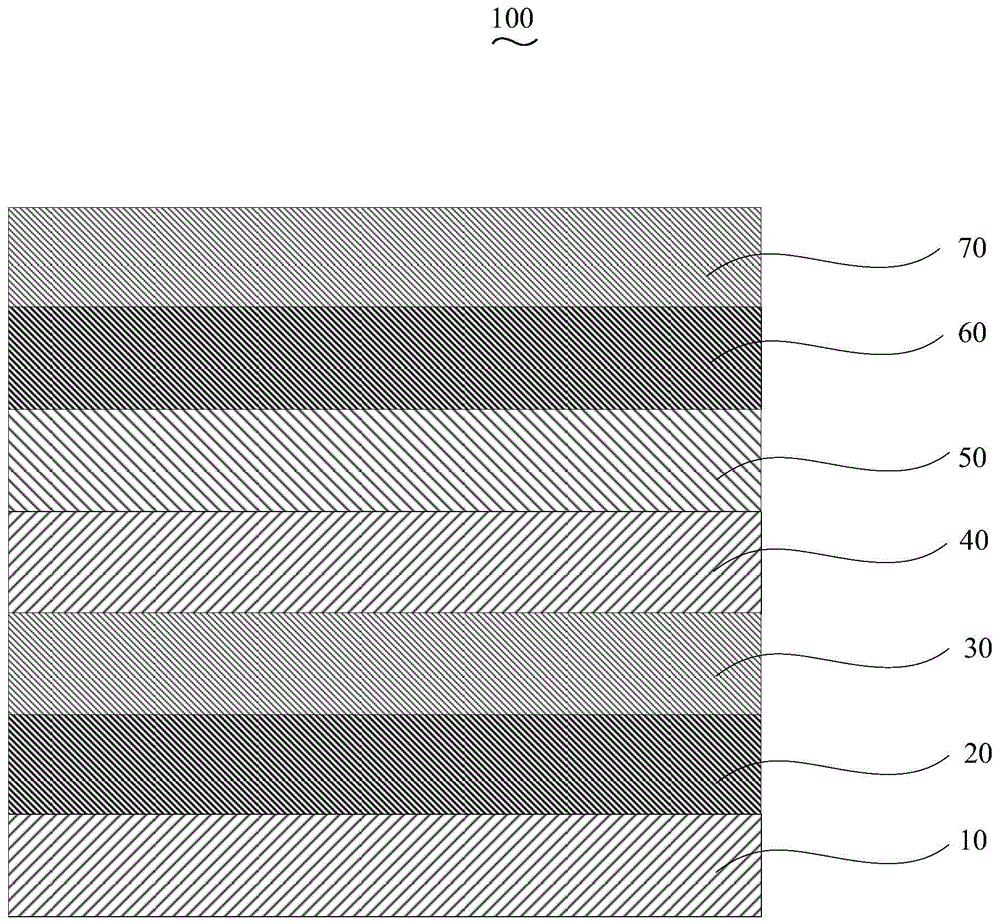
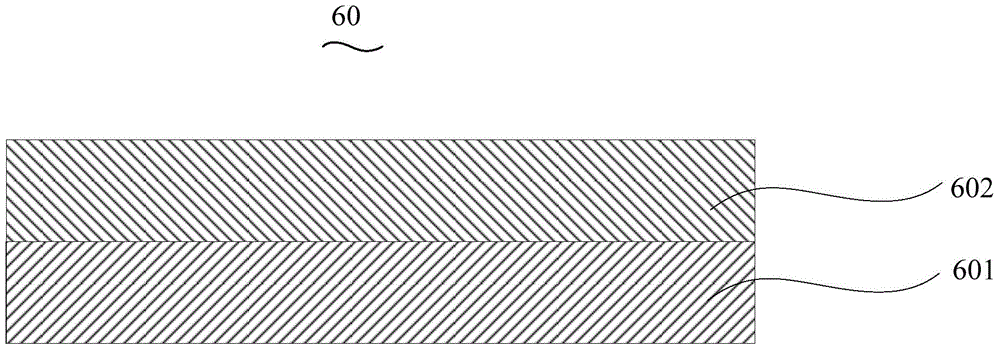
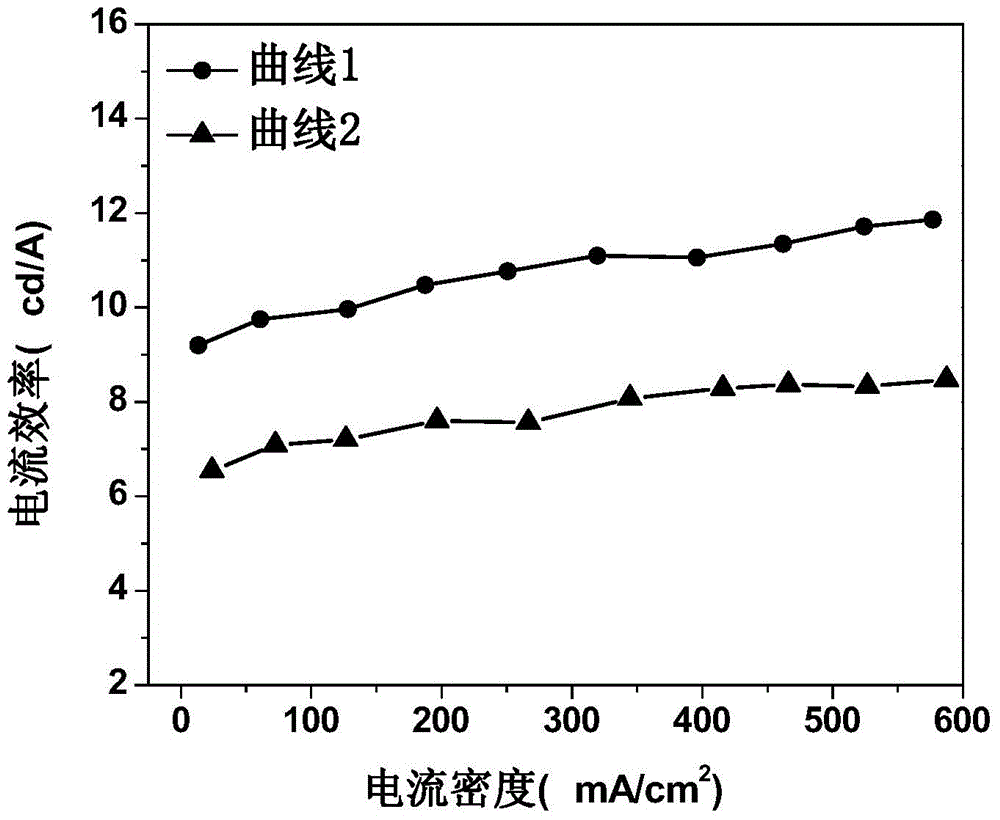
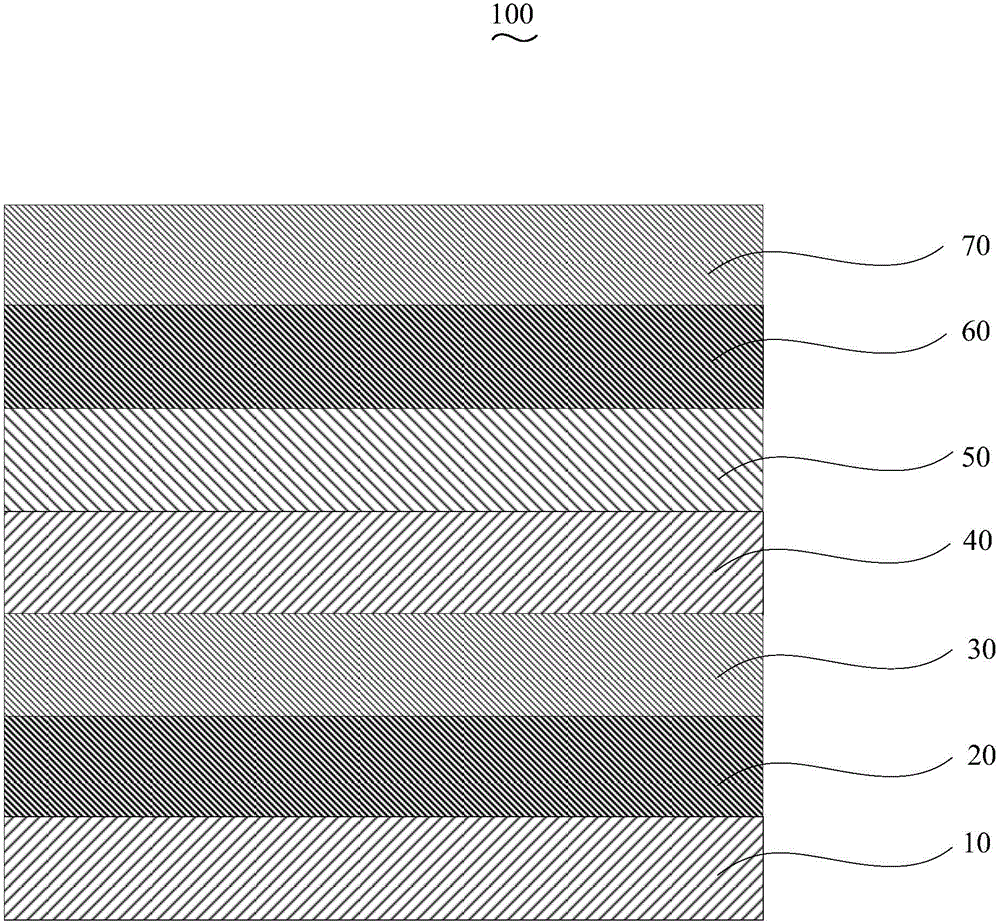
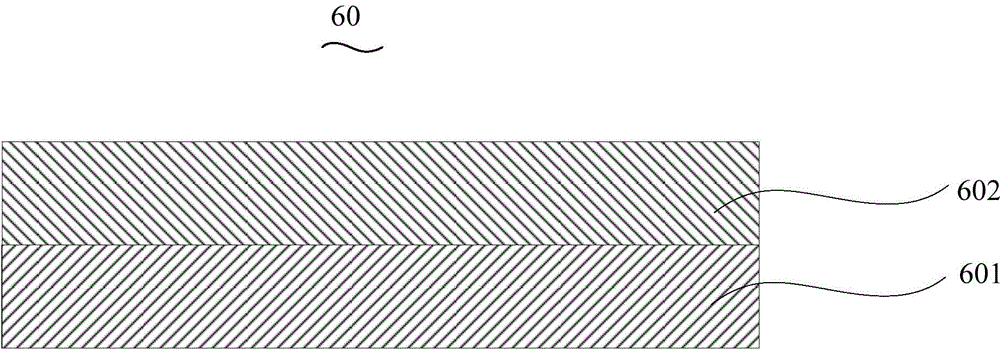
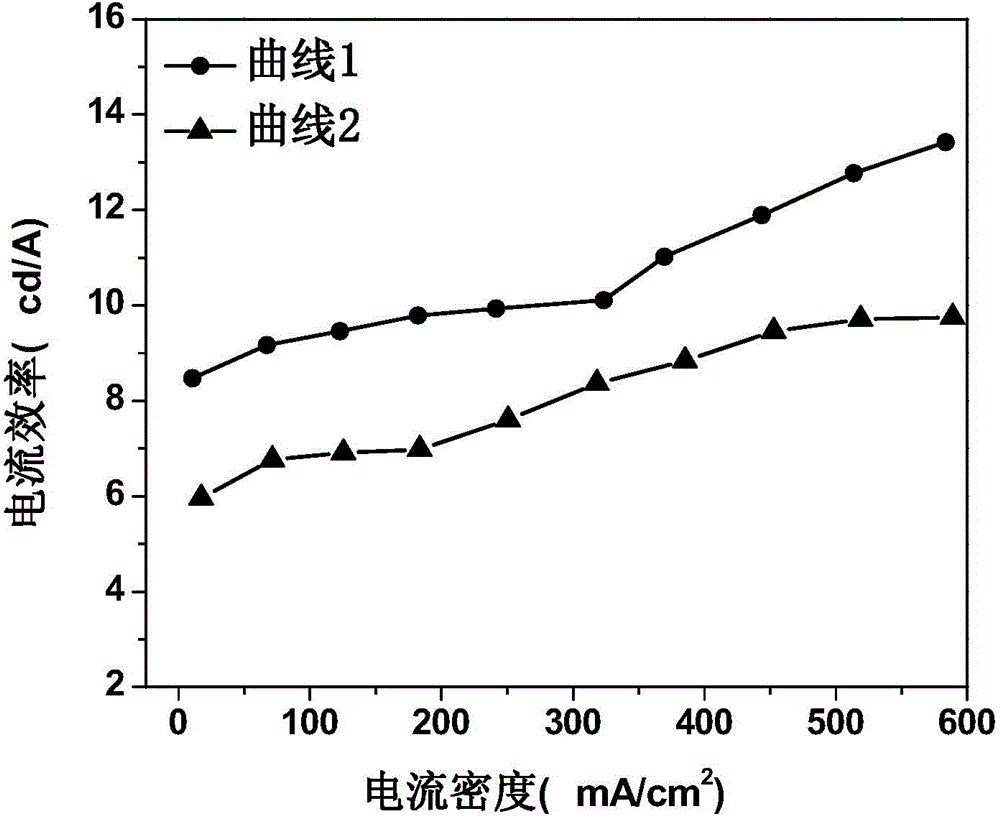
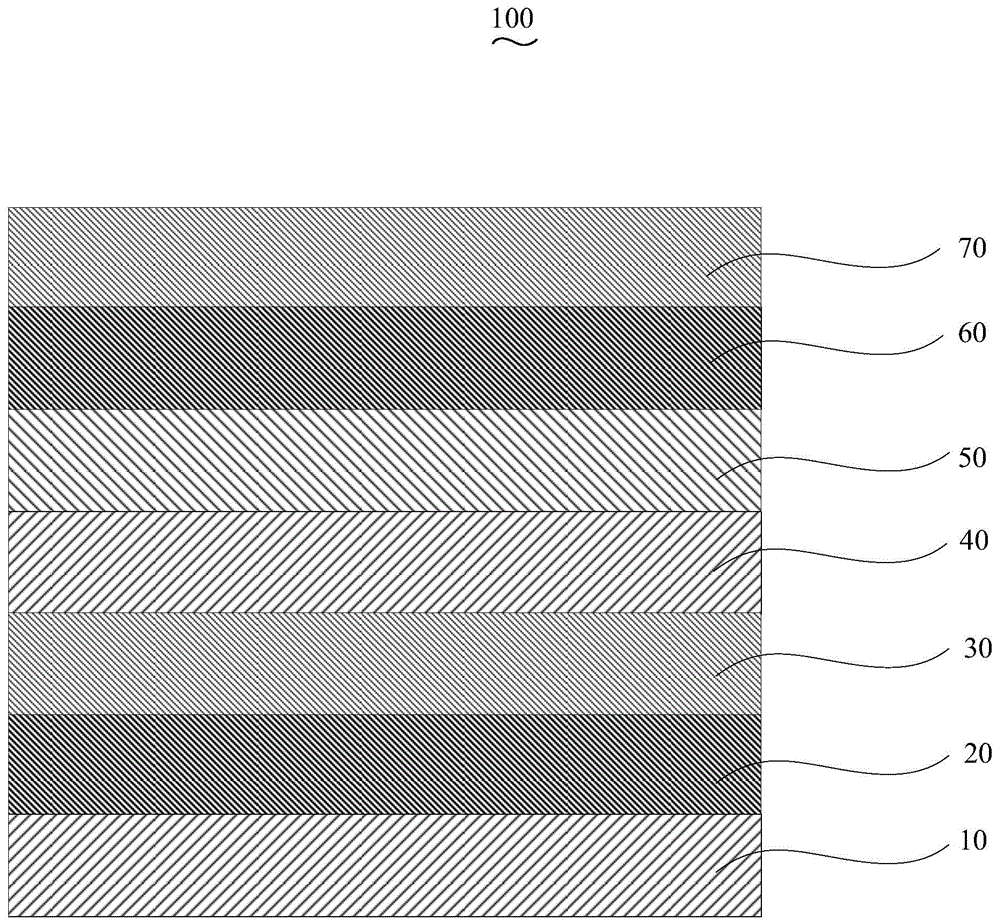
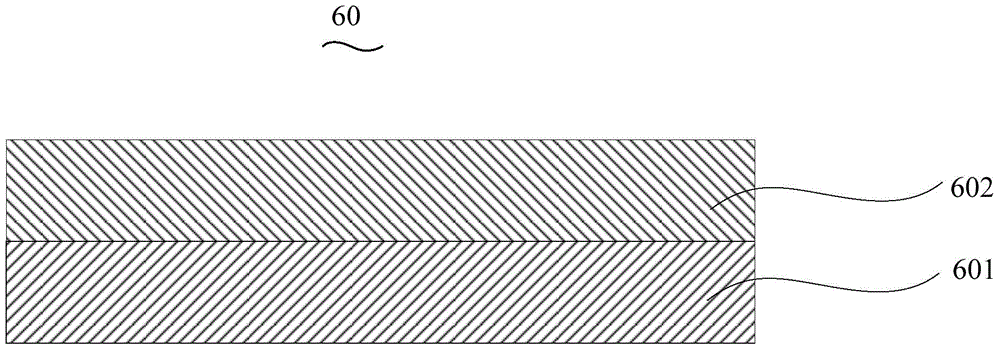
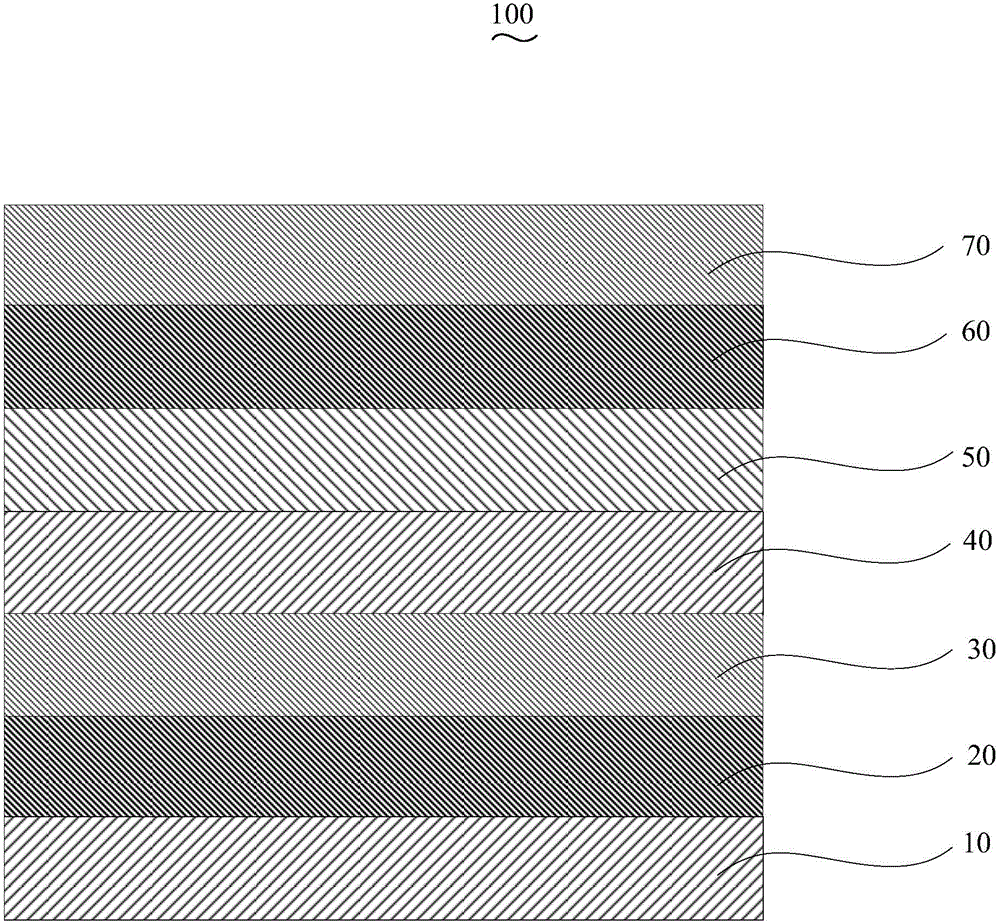
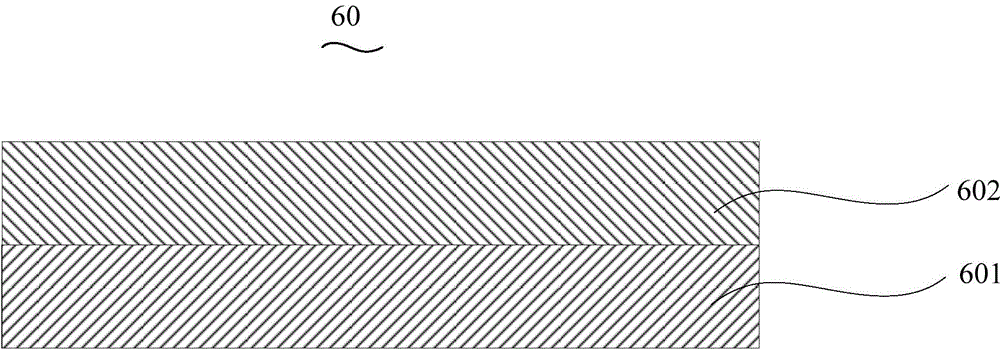
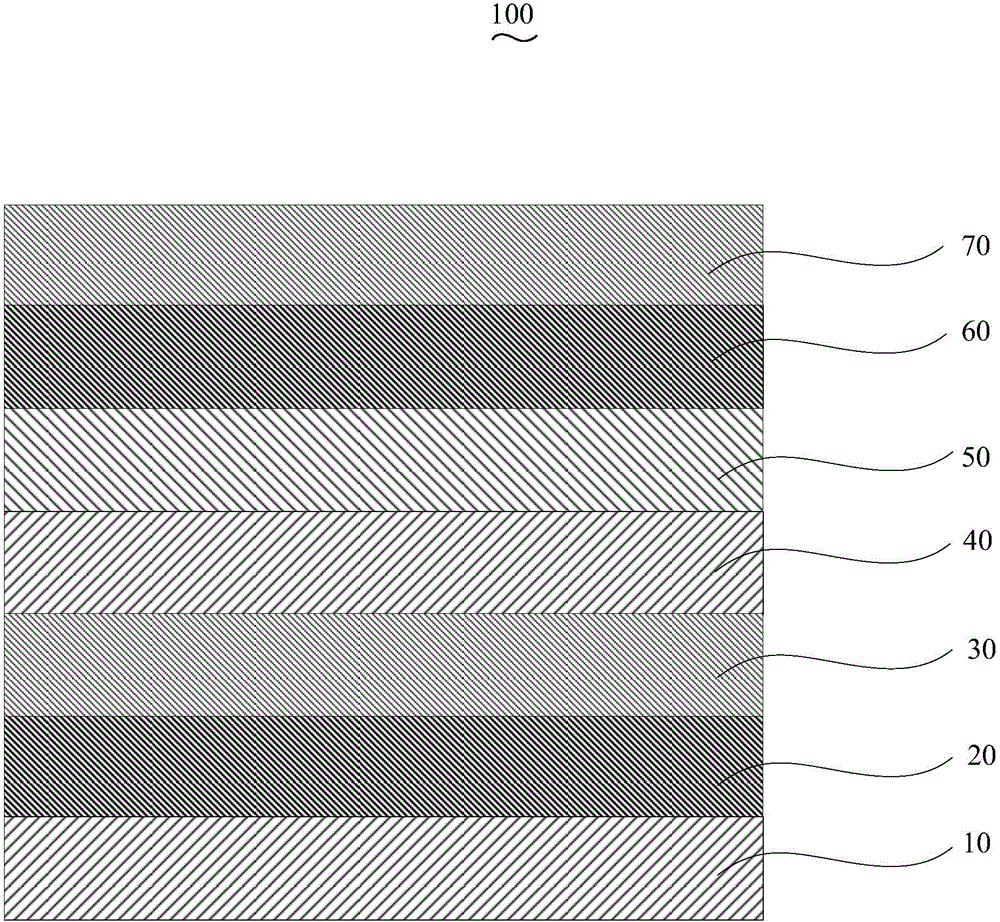
Organic electroluminescent device and manufacturing method thereof
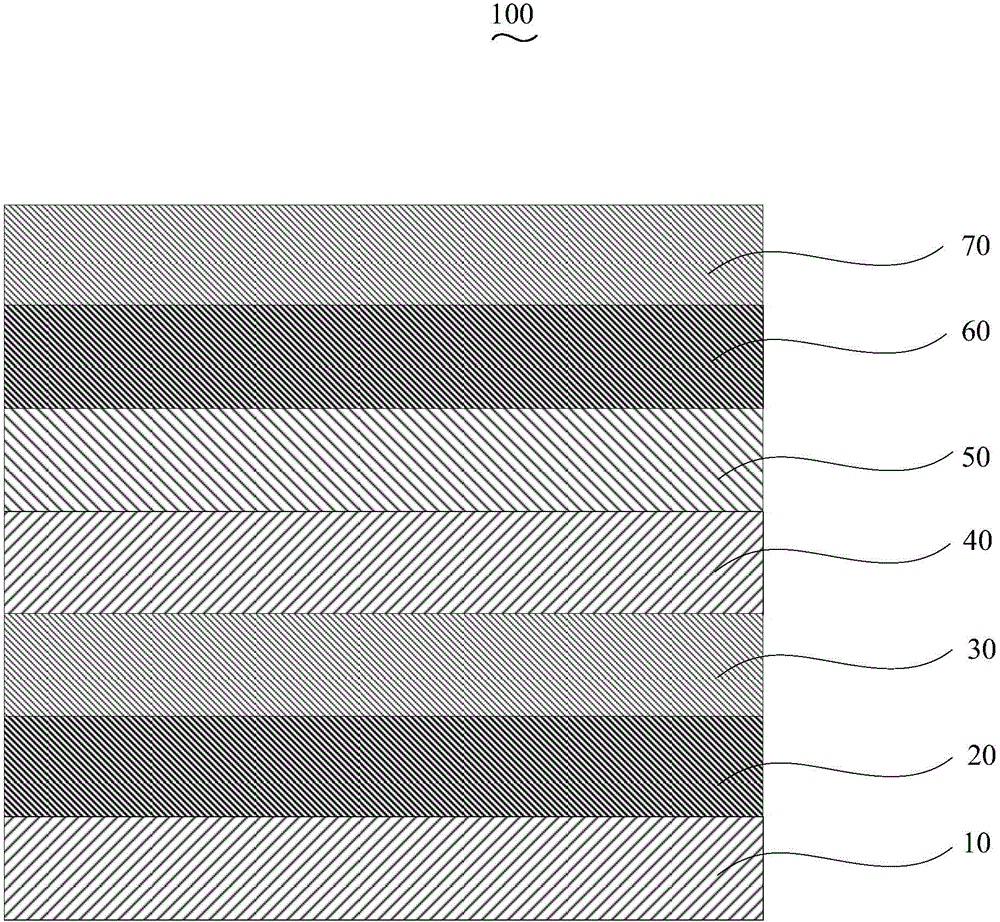
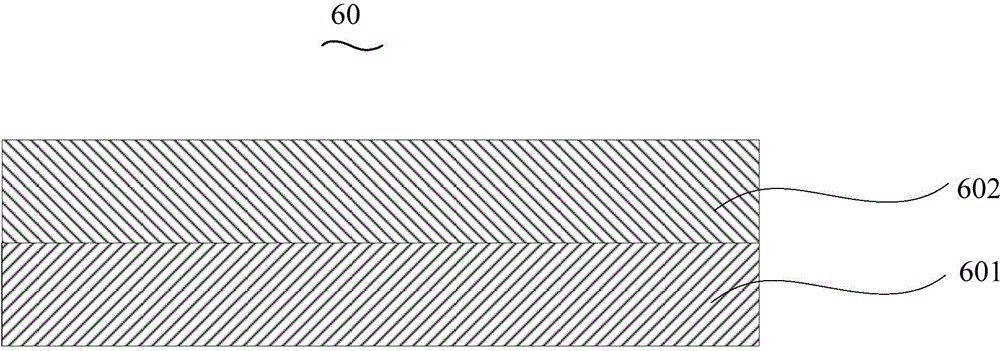
InactiveCN104659227AEasy injectionIncrease transfer rateSolid-state devicesSemiconductor/solid-state device manufacturingRubidium sulfateRubidium carbonate
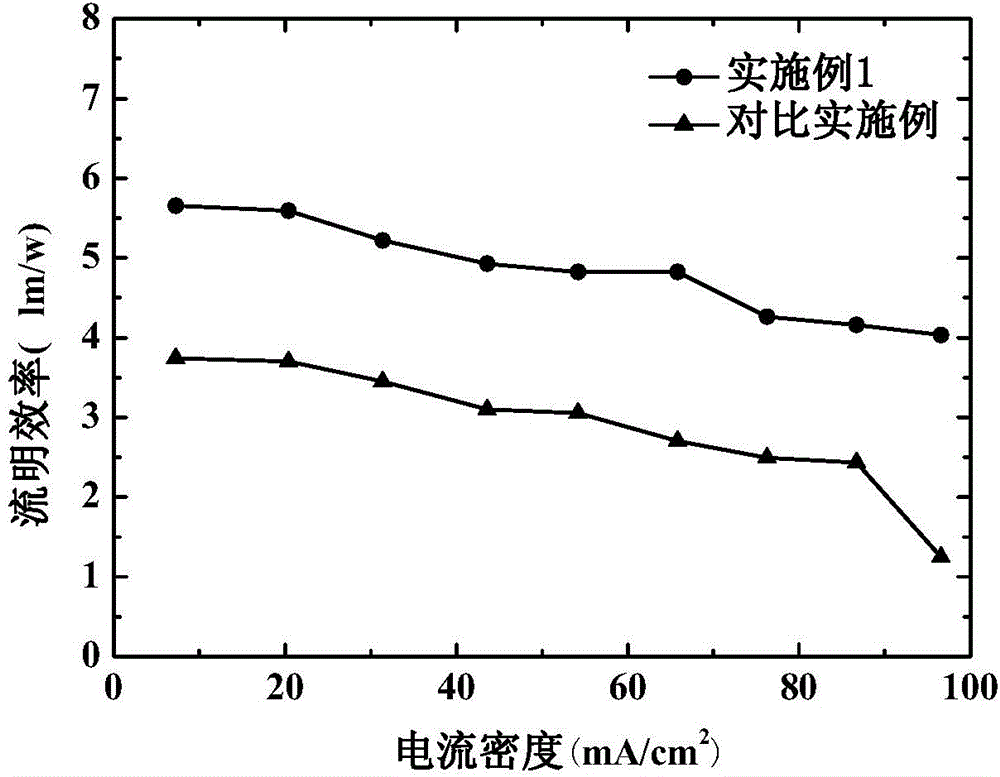
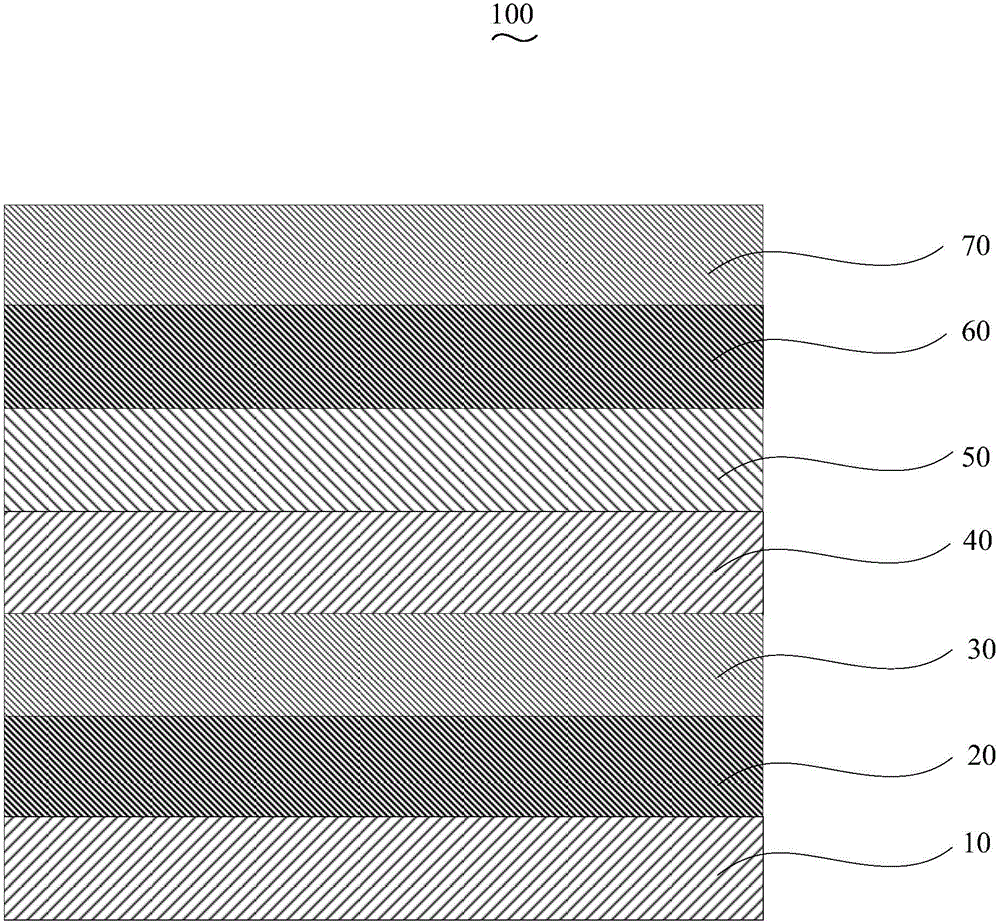
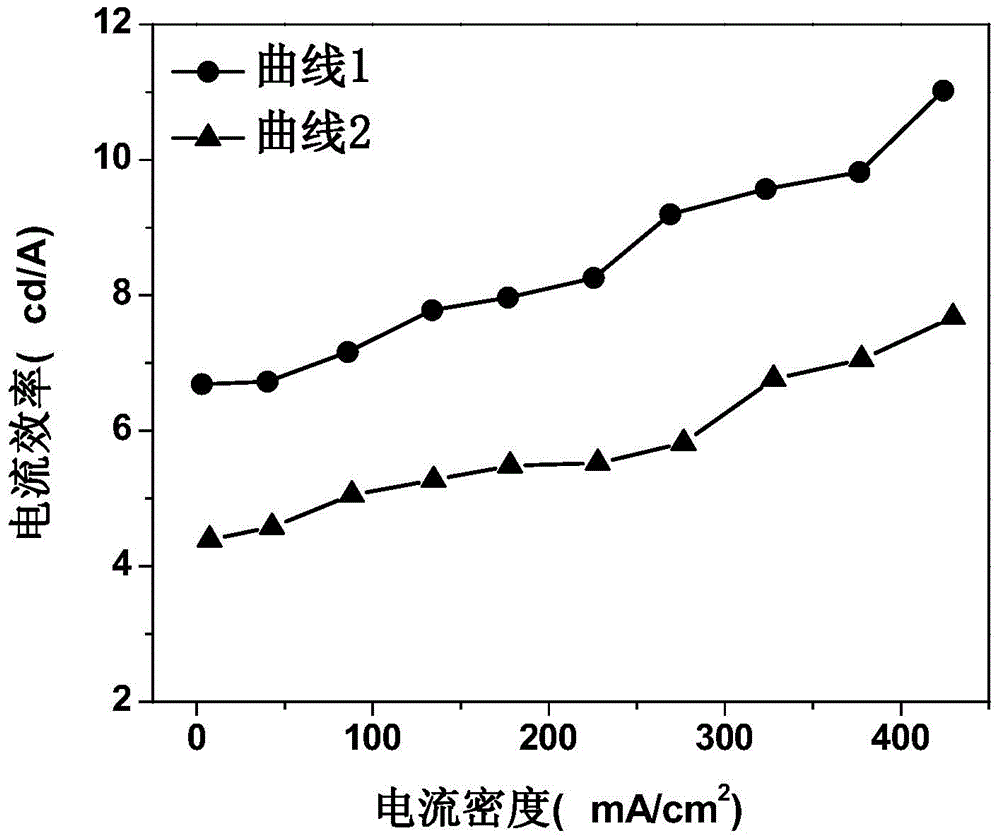
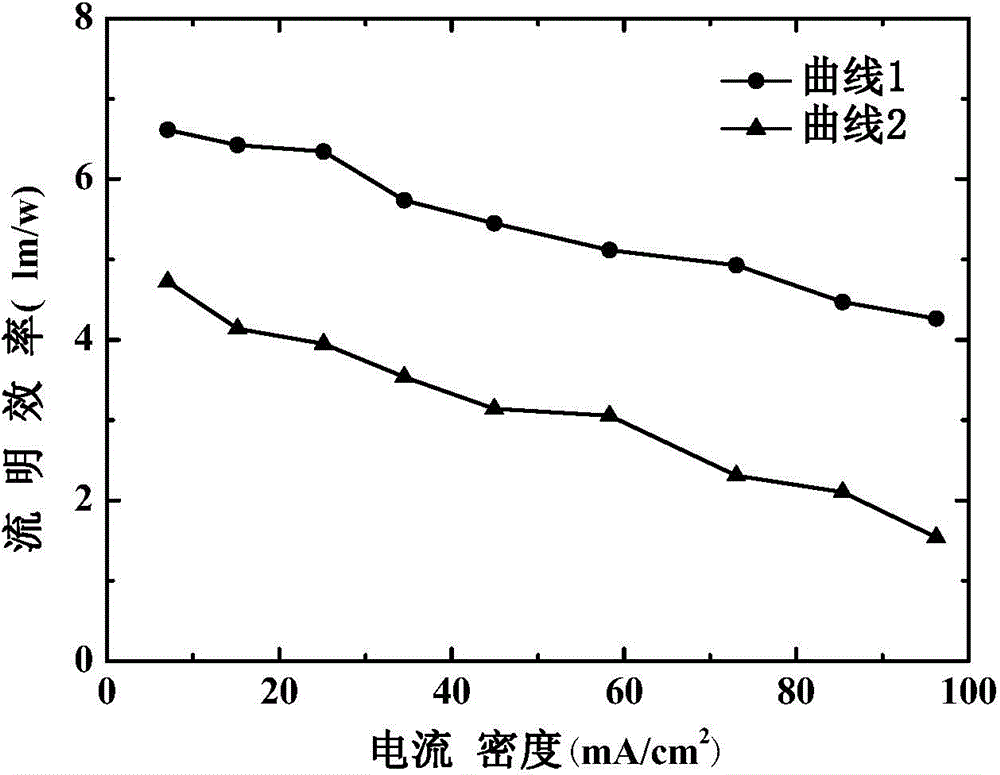
The invention discloses an organic electroluminescent device and a manufacturing method thereof. The organic electroluminescent device comprises a glass substrate, a conductive anode, a hole injection layer, a hole transmission layer, a luminous layer, an electron transmission layer, an electron injection layer and a cathode which are stacked in sequence, wherein the electron injection layer comprises a rhenium compound doped layer, a rubidium compound layer and a metal layer which are stacked in sequence; the rhenium compound doped layer is arranged on the surface of the electron transmission layer and made from a mixed material formed by mixing a rhenium compound with a bipolar organic material at a mass ratio of (1:1) to (4:1); the rubidium compound layer is made from rubidium carbonate, rubidium chloride, rubidium nitrate or rubidium sulfate; the metal layer is made from low-work-function metal with the work function ranging from -2.0 eV to -3.5 eV. The electron injection layer can effectively improve the luminous efficiency of the device.
Owner:OCEANS KING LIGHTING SCI&TECH CO LTD +2
Organic electroluminescence device and preparation method thereof
InactiveCN104659277ALower injection barrierImprove injection efficiencySolid-state devicesSemiconductor/solid-state device manufacturingRubidium compoundGlass transition
An organic electroluminescence device comprises an anode, a hole injection layer, a hole transport layer, a luminescent layer, an electron transport layer, an electron injection layer and a cathode which are stacked in sequence, wherein the electron injection layer consists of a ternary doped layer and a cesium salt doped layer; the ternary doped layer comprises a rubidium compound material, a zinc oxide material and an electron transport material I; the rubidium compound material is at least one of rubidium carbonate, rubidium chloride, rubidium nitrate and rubidium sulfate; the glass transition temperature of the electron transport material I is 50-100 DEG C; the cesium salt doped layer comprises a cesium salt material and an electron transport material II doped in the cesium salt material; the glass transition temperature of the electron transport material II is 50-100 DEG C; the cesium salt material is at least one of cesium fluoride, cesium carbonate, cesium azide and cesium chloride. The organic electroluminescence device is relatively high in luminescence efficiency. The invention further provides a preparation method of the organic electroluminescence device.
Owner:OCEANS KING LIGHTING SCI&TECH CO LTD +2
Method for preparing Nano tube of titanate
InactiveCN100455517CLarge specific surface areaPolycrystalline material growthTitanium compoundsCopper nitrateSodium titanate
Owner:HENAN UNIVERSITY
Organic electroluminescence device and preparation method thereof
InactiveCN104659251AIncrease transfer rateLower injection barrierSolid-state devicesSemiconductor/solid-state device manufacturingRubidium compoundIodide
An organic electroluminescence device comprises an anode, a hole injection layer, a hole transporting layer, a luminous layer, an electron transporting layer, an electron injection layer and a cathode which are overlapped in sequence, wherein the electron injection layer is made of one or more rubidium compound materials, one or more copper oxide materials and one or more of metal materials; the rubidium compound material(s) is / are one or more of rubidium carbonate, rubidium chloride, rubidium nitrate and rubidium sulfate; the copper oxide material(s) is / are one or more of cuprous iodide, cuprous oxide, copper phthalocyanine and cupric oxide; the work function of the metal materials is -4.0 eV to -5.5 eV. The light efficiency of the organic electroluminescence device is relatively high. The invention further provides a preparation method of the organic electroluminescence device.
Owner:OCEANS KING LIGHTING SCI&TECH CO LTD +2
Cesium-rubidium-potassium monolithic flameproof glass and preparation method
ActiveCN102503178BImprove surface structureTight surface structurePotassium fluoridePotassium hydroxide
Owner:SHENYANG JIANZHU UNIVERSITY
Organic electroluminescence device and preparation method thereof
InactiveCN104659219ALower injection barrierImprove injection efficiencySolid-state devicesSemiconductor/solid-state device manufacturingSodium bicarbonateRubidium compound
An organic electroluminescence device comprises an anode, a hole injection layer, a hole transport layer, a luminescent layer, an electron transport layer, an electron injection layer and a cathode which are stacked in sequence, wherein the electron injection layer consists of a rubidium compound doped layer and a passive material layer; the rubidium compound doped layer comprises a rubidium compound material and a sodium salt material doped in the rubidium compound material; the rubidium compound material is at least one of rubidium carbonate, rubidium chloride, rubidium nitrate and rubidium sulfate; the sodium salt material is at least one of sodium carbonate, sodium chloride, sodium bicarbonate and sodium fluoride; the passive material layer is made at least one of silicon dioxide, aluminum oxide, nickel oxide and copper oxide. The organic electroluminescence device is relatively high in luminescence efficiency. The invention further provides a preparation method of the organic electroluminescence device.
Owner:OCEANS KING LIGHTING SCI&TECH CO LTD +2
Process for preparing rubidium-doped titanium dioxide photocatalytic material by virtue of spray drying method
InactiveCN109939666AImprove photocatalytic efficiencyImprove quantum efficiencyCatalyst activation/preparationMetal/metal-oxides/metal-hydroxide catalystsAcetic acidRubidium nitrate
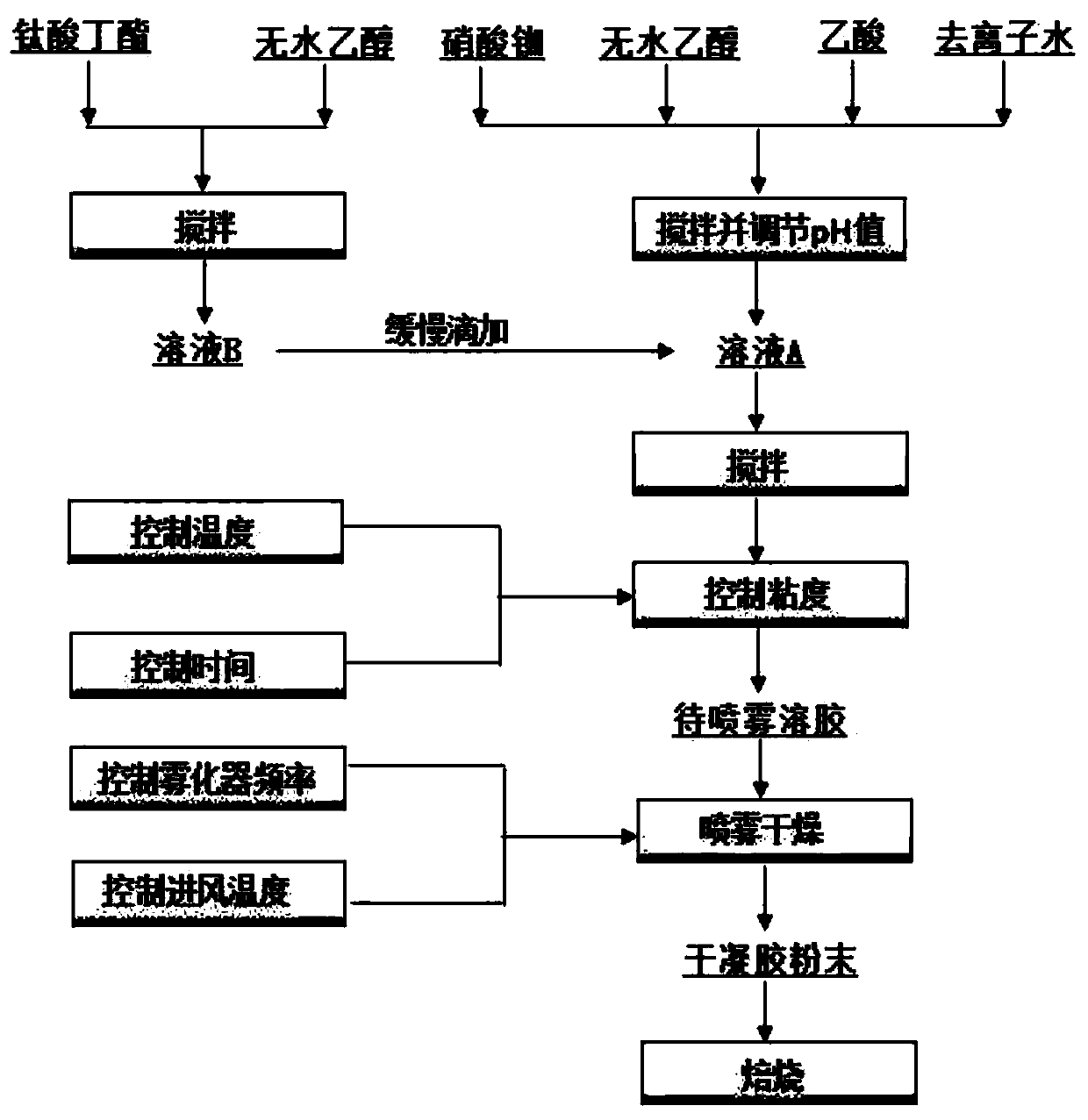
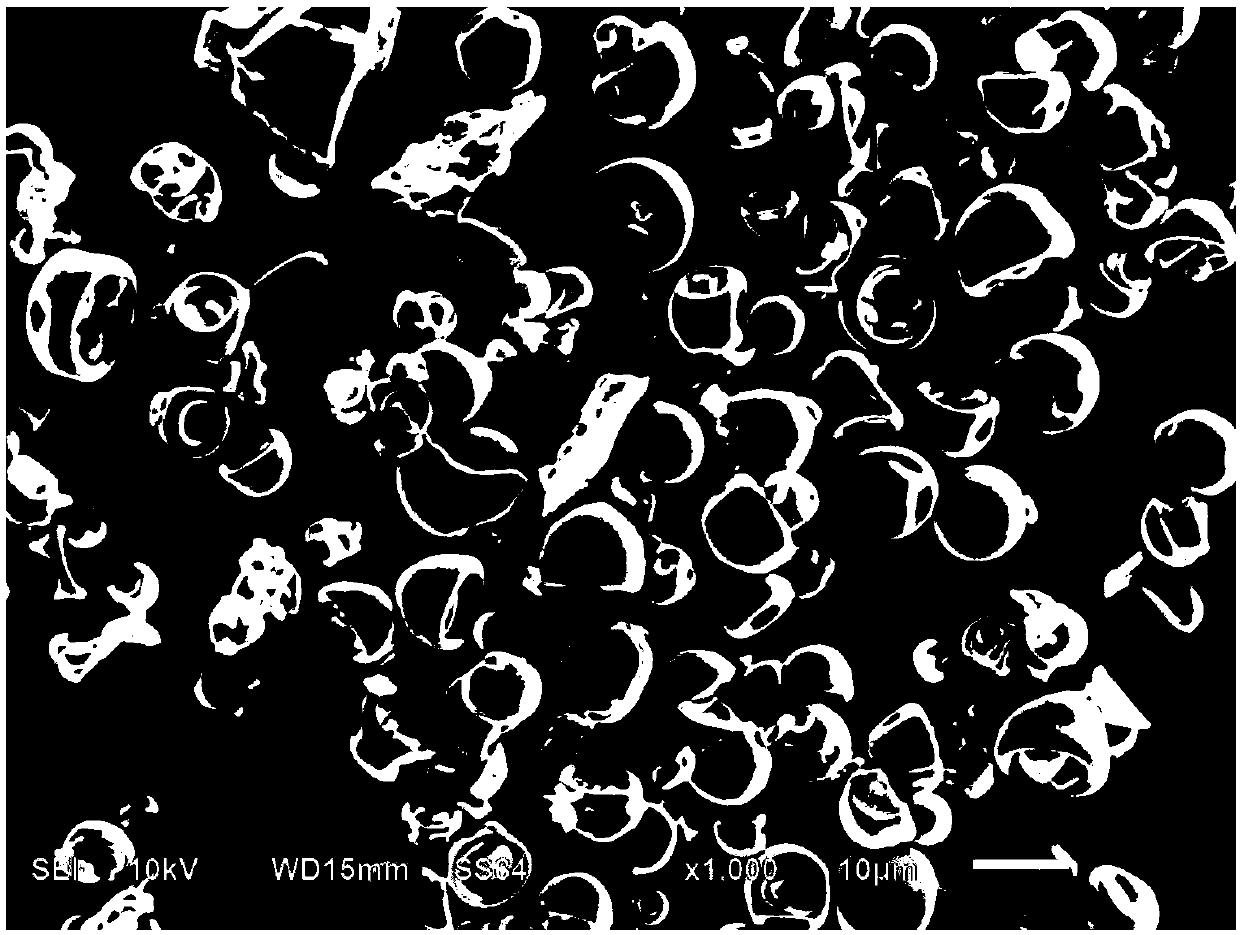
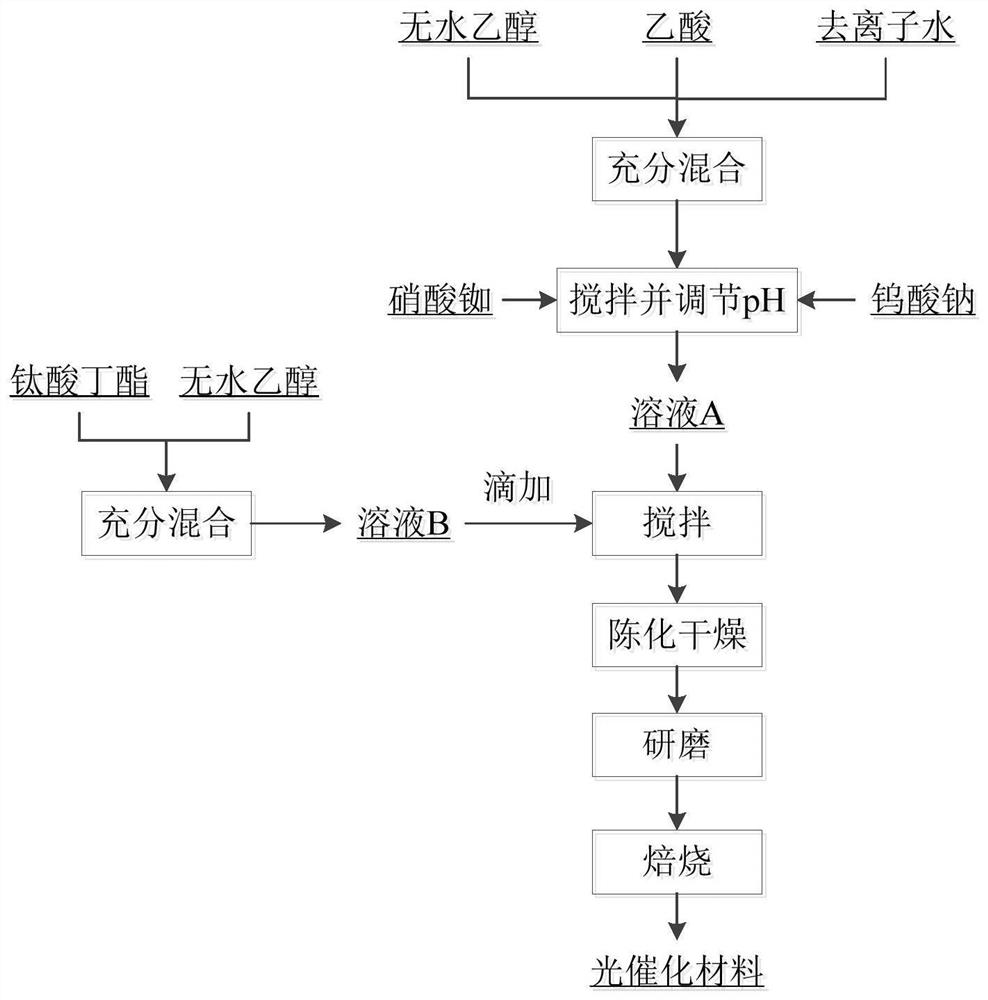
The invention discloses a process for preparing rubidium-doped titanium dioxide photocatalytic material by virtue of a spray drying method. The process comprises the following steps: (1) preparing a rubidium nitrate solution, adding absolute ethyl alcohol and acetic acid, adding nitric acid to adjust the pH value to 1.0-3.0, and adequately stirring at the room temperature, so as to obtain a solution A; (2) adequately stirring ethanol with butyl titanate at the room temperature, so as to prepare a solution B; (3) slowly dropwise adding the solution B into the solution A, and stirring at 20-35 DEG C, so as to obtain sol with viscosity controlled at 20mPa.s-100mPa.s; (4) carrying out spray drying on the sol by virtue of a centrifugal atomizer; and (5) roasting the dried material, so as to obtain the photocatalytic material. By virtue of the process, the rubidium-doped titanium dioxide photocatalytic material with high photocatalytic activity can be obtained.
Owner:有研资源环境技术研究院(北京)有限公司
Growth promotion regulator after peach grafting
InactiveCN108283186AImprove disease resistanceImprove developmentBiocidePlant growth regulatorsAdditive ingredientHydrolysate
The invention discloses a growth promotion regulator after peach grafting. The regulator comprises the following raw materials in parts by weight: 2-6 parts of seaweed extract, 1-4 parts of sucrose laurate, 1-5 parts of 5-nitroguaiacol sodium salt, 2-6 parts of pyridoxine hydrochloride, 3-7 parts of hexanoic-2-(diethylamino) ethyl estercitrate, 6-9 parts of thiophanate methyl, 7-10 parts of lactoalbumin hydrolysate, 15-20 parts of cordate houttuynia dry powder, 4-7 parts of ethychlozate, 2-5 parts of amino-oligosaccharin, 1-3 parts of rubidium nitrate, 6-9 parts of pine needle nutrient solution and 3-6 parts of diethyl aminoethyl hexanoate. The regulator can adjust and balance nutrition conduction in a plant body, promotes root system growth, accelerates a plant to absorb nutrition ingredients, significantly increases a chlorophyll content, improves photosynthesis, promotes metabolism, and therefore, achieves effects of improving product quality, boosting a yield and improving diseaseresistance of the plant.
Owner:崔伟
Organic electroluminescence device and preparation method thereof
InactiveCN104659246AImprove conductivityImprove light extraction efficiencySolid-state devicesSemiconductor/solid-state device manufacturingIron(III) sulfideRubidium compound
An organic electroluminescence device comprises an anode, a hole injection layer, a hole transporting layer, a luminous layer, an electron transporting layer, an electron injection layer and a cathode which are overlapped in sequence, wherein the electron injection layer comprises a rubidium compound doped layer and a metal doped layer; the rubidium compound doped layer is made of a rubidium compound material and a molysite material doped in the rubidium compound material; the rubidium compound material is one or more of rubidium carbonate, rubidium chloride, rubidium nitrate and rubidium sulfate; the molysite material is one or more of ferric chloride, ferric bromide and ferric sulfide; the metal doped layer is made of a first metal material and a second metal material doped in the first metal material; the work function of the first metal material is -2.0 eV to -3.5 eV; the work function of the second metal material is -4.0 eV to -5.5 eV. The light efficiency of the organic electroluminescence device is relatively high. The invention further provides a preparation method of the organic electroluminescence device.
Owner:OCEANS KING LIGHTING SCI&TECH CO LTD +2
Organic light-emitting device and manufacturing method thereof
InactiveCN104659215AEasy injectionIncrease transfer rateSolid-state devicesSemiconductor/solid-state device manufacturingRubidium compoundPhenanthroline
The invention discloses an organic light-emitting device, which comprises an anode, a hole injection layer, a hole transmission layer, a light emitting layer, an electron transport layer, an electron injection layer and a cathode laminated in sequence, wherein the electron injection layer is composed of a two-element doping layer and a rubidium compound layer; the two-element doping layer rubidium compound material and electron transport material; the rubidium compound material selects at least one from rubidium carbonate, rubidium chloride, rubidium nitrate and rubidium sulfate; the electron transport material selects at least one from 4, 7-Diphenyl-1, 10-phenanthroline, 2-(4-tert-Butylphenyl)-5-(4-biphenyl)-1,3,4-oxadiazole, 8-Hydroxyquinoline aluminum salt and N-aryl benzimidazole; and the rubidium compound layer material is also the rubidium compound material. The above organic light-emitting device is high in light emitting efficiency. The invention also provides an organic light-emitting device manufacturing method.
Owner:OCEANS KING LIGHTING SCI&TECH CO LTD +2
Organic electroluminescence device and preparation method thereof
InactiveCN104659253AIncrease transfer rateLow melting pointSolid-state devicesSemiconductor/solid-state device manufacturingRubidium compoundAzide
An organic electroluminescence device comprises an anode, a hole injection layer, a hole transporting layer, a luminous layer, an electron transporting layer, an electron injection layer and a cathode which are overlapped in sequence, wherein the electron injection layer is made of one or more rubidium compound materials, one or more cesium salt materials and titanium dioxide; the rubidium compound material(s) is / are one or more of rubidium carbonate, rubidium chloride, rubidium nitrate and rubidium sulfate; the cesium salt material(s) is / are one or more of cesium fluoride, cesium carbonate, cesium azide and cesium chloride. The light efficiency of the organic electroluminescence device is relatively high. The invention further provides a preparation method of the organic electroluminescence device.
Owner:OCEANS KING LIGHTING SCI&TECH CO LTD +2
Organic electroluminescence device and preparation method thereof
InactiveCN104659224AIncrease transfer rateImprove injection efficiencySolid-state devicesSemiconductor/solid-state device manufacturingLithium oxideLithium chloride
An organic electroluminescence device comprises an anode, a hole injection layer, a hole transport layer, a luminescent layer, an electron transport layer, an electron injection layer and a cathode which are stacked in sequence, wherein the electron injection layer consists of a rubidium compound doped layer and a metal doped layer; the rubidium compound doped layer comprises a rubidium compound material and a metal material I doped in the rubidium compound material; the rubidium compound material is at least one of rubidium carbonate, rubidium chloride, rubidium nitrate and rubidium sulfate; the work function of the metal material I is -2.0 eV to -3.5 eV; the metal doped layer comprises a metal material II and a lithium salt material doped in the metal material II; the work function of the metal material II is -2.0 eV to -3.5 eV; the lithium salt material is at least one of lithium oxide, lithium fluoride, lithium chloride and lithium bromide. The organic electroluminescence device is relatively high in luminescence efficiency. The invention further provides a preparation method of the organic electroluminescence device.
Owner:OCEANS KING LIGHTING SCI&TECH CO LTD +2
Organic electroluminescence device and preparation method thereof
InactiveCN104659222AEasy injectionIncrease transfer rateSolid-state devicesSemiconductor/solid-state device manufacturingRubidium compoundPhenanthroline
An organic electroluminescence device comprises an anode, a hole injection layer, a hole transporting layer, a luminous layer, an electron transporting layer, an electron injection layer and a cathode which are overlapped in sequence, wherein the electron injection layer comprises a two-element-doped layer and a metal doped layer; the two-element-doped layer is made of one or more rubidium compound materials and an organosilicon small molecular material; the rubidium compound material(s) is / are one or more of rubidium carbonate, rubidium chloride, rubidium nitrate and rubidium sulfate; the energy gap of the organosilicon small molecular material is -3.5 eV to -5.5 eV; the metal doped layer is made of one or more of metal materials and one or more electron transporting materials; the work function of the metal materials is -2.0 eV to -3.5 eV; the electron transporting material(s) is / are one or more of 4,7-diphenyl-1,10-phenanthroline, 2-(4'-tert-butylphenyl)-5-(4'-biphenyl)-1,3,4-oxadiazole, 8-hydroxyquinoline aluminum, and N-aryl benzimidazole.
Owner:OCEANS KING LIGHTING SCI&TECH CO LTD +2
A kind of ultra-thin chemically strengthened glass and preparation method thereof
ActiveCN108675652BAchieve chemical strengtheningImprove exchange efficiencyRubidium nitratePotassium nitrate
Owner:WUJIANG GOLDEN GLASS TECH +1
Rubidium and tungsten co-doped titanium dioxide photocatalytic material and preparation method thereof
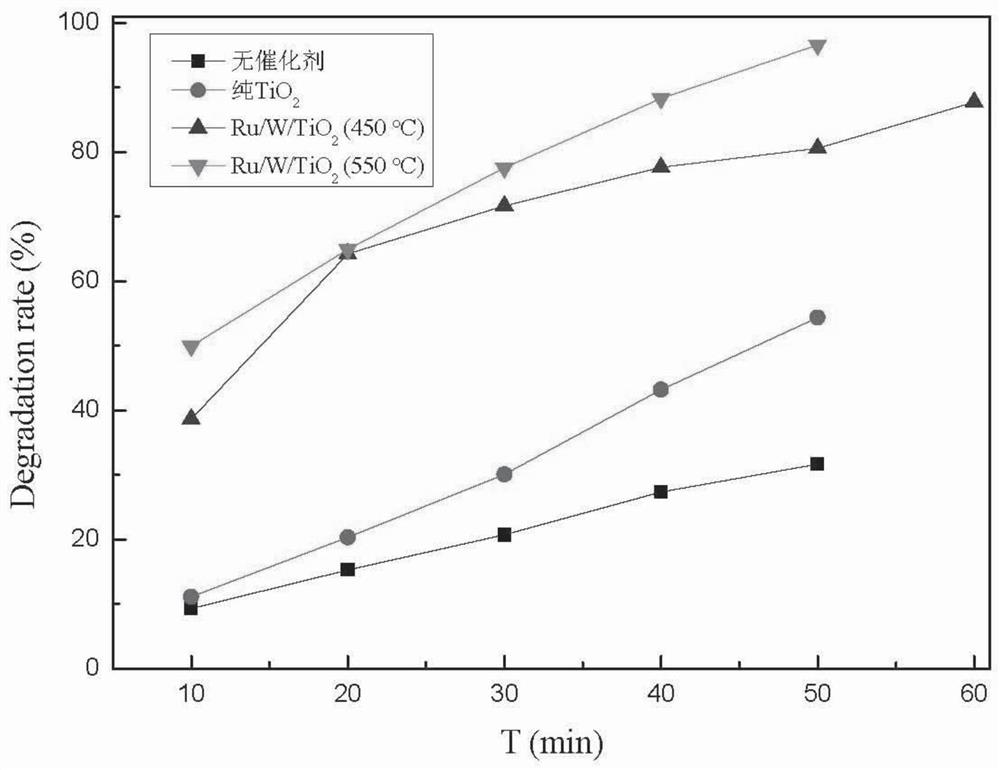
PendingCN113117658AReduce chance of recombinationImprove photocatalytic degradation performanceWater treatment compoundsWater contaminantsRubidium nitrateAcetic acid
The invention discloses a rubidium and tungsten co-doped titanium dioxide photocatalytic material and a preparation method thereof. According to the photocatalytic material, titanium dioxide is doped with rubidium and tungsten elements, and the molar doping ratio of the rubidium and tungsten elements is 0.01%-5.0%. The photocatalytic material is grey white powder. The preparation method comprises the following steps: (1) uniformly mixing absolute ethyl alcohol, acetic acid and deionized water, adding rubidium nitrate and sodium tungstate into the mixture, fully dissolving, and regulating the pH value of the solution to 0.5-7.0 by using nitric acid to obtain a solution A; (2) dissolving butyl titanate in absolute ethyl alcohol of which the volume is equal to that of the absolute ethyl alcohol in the step (1) to obtain a solution B; (3) slowly dropwise adding the solution B into the rapidly stirred solution A, continuously stirring after dropwise adding until gel is formed, and aging and air-drying the gel; and (4) grinding the obtained xerogel into powder, and roasting the powder in a muffle furnace to obtain the rubidium and tungsten co-doped titanium dioxide photocatalytic material.
Owner:有研资源环境技术研究院(北京)有限公司 +1
Organic light-emitting device and manufacturing method thereof
InactiveCN104659216AEasy to injectEasy injectionSolid-state devicesSemiconductor/solid-state device manufacturingRubidium compoundMetallic materials
The invention discloses an organic light-emitting device, which comprises an anode, a hole injection layer, a hole transmission layer, a light emitting layer, an electron transport layer, an electron injection layer and a cathode laminated in sequence, wherein the electron injection layer is composed of a three-element doping layer and a titanium dioxide layer; the three-element doping layer comprises rubidium compound material, electron transport material and metal material; the rubidium compound material selects at least one from rubidium carbonate, rubidium chloride, rubidium nitrate and rubidium sulfate; and the electron transport material selects at least one from 4, 7-Diphenyl-1, 10-phenanthroline, 2-(4-tert-Butylphenyl)-5-(4-biphenyl)-1,3,4-oxadiazole, 2,9-dimethyl-4,7-diphenyl-1,10-phenanthroline, and 2,2'-(1,3-Phenylene)-bis [5-(4-tert-butylphenyl)-1,3,4-oxadiazole]. The above organic light-emitting device is high in light emitting efficiency. The invention also provides an organic light-emitting device manufacturing method.
Owner:OCEANS KING LIGHTING SCI&TECH CO LTD +2
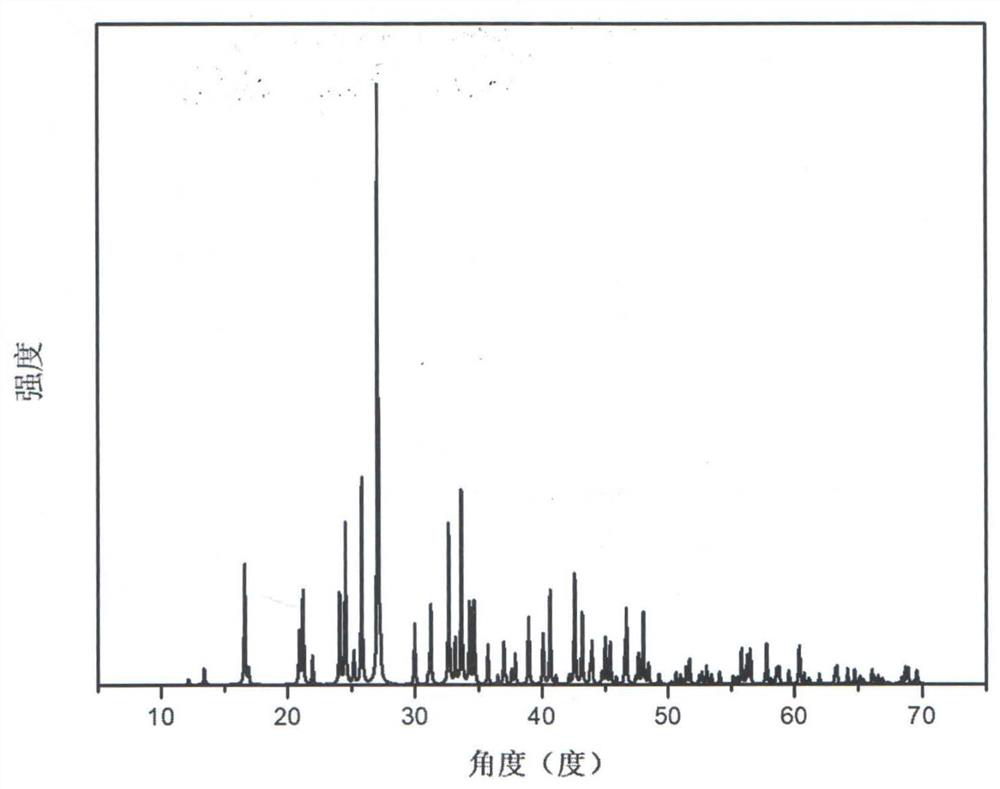
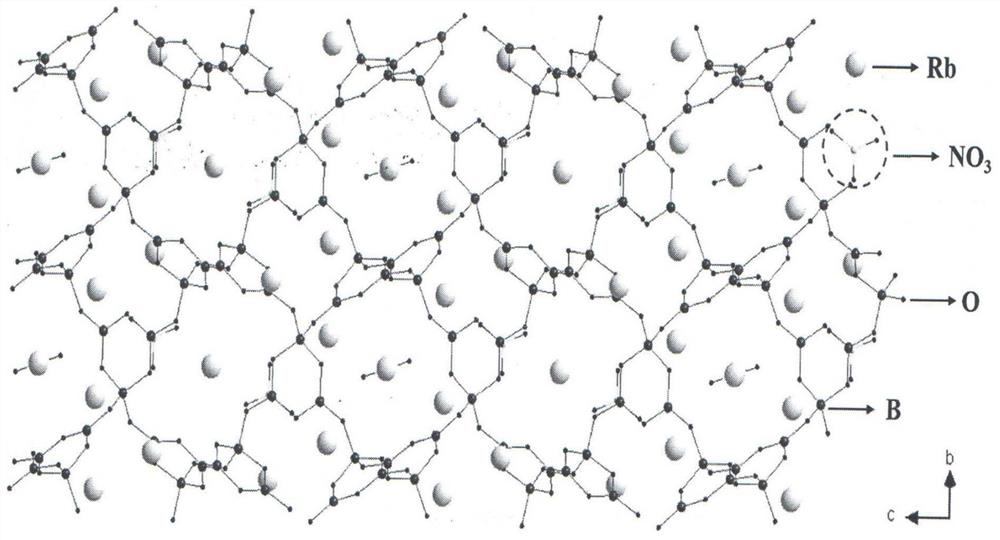
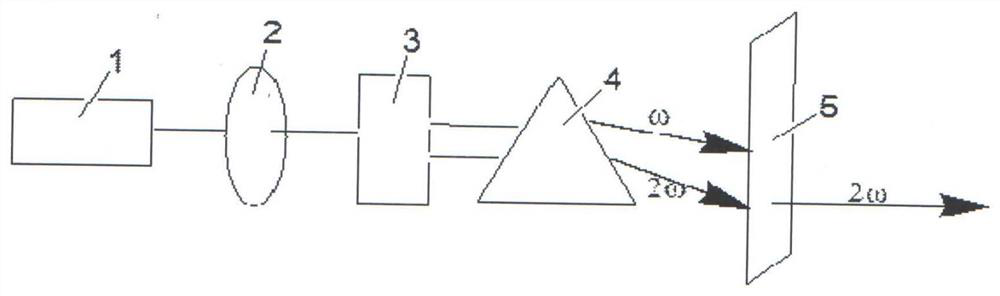
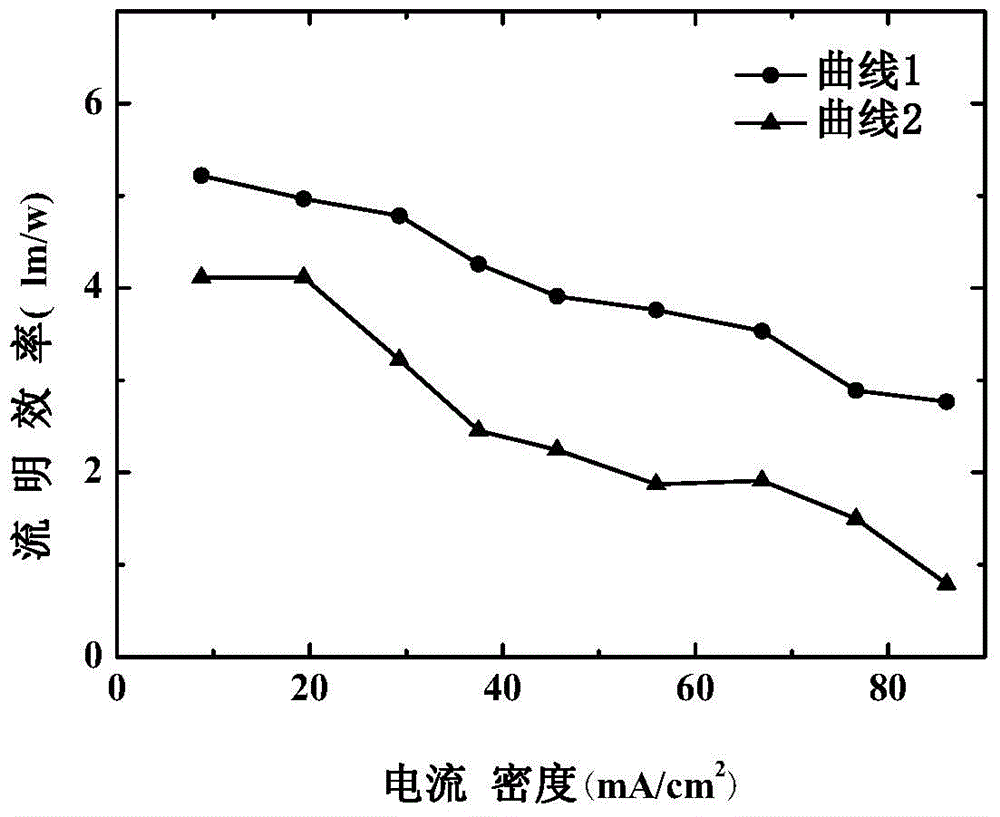
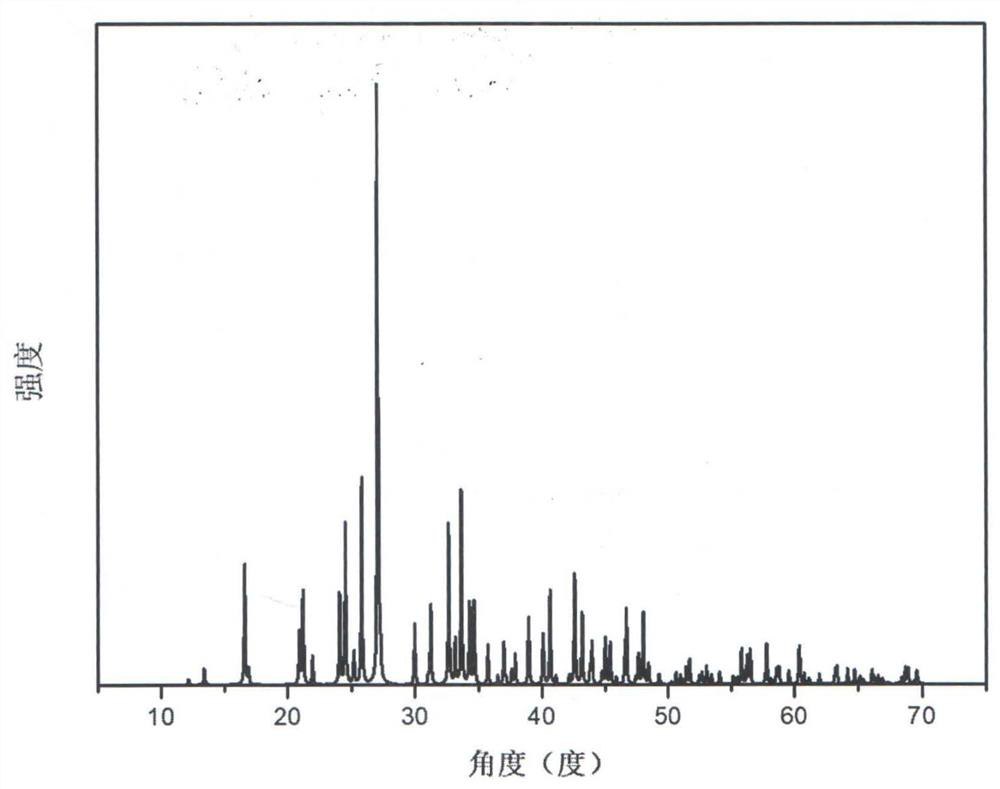
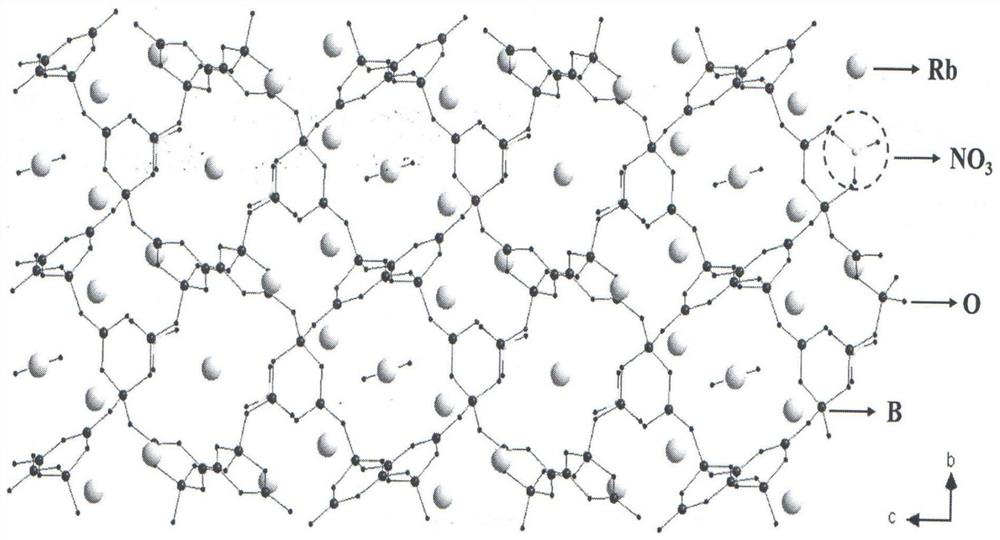
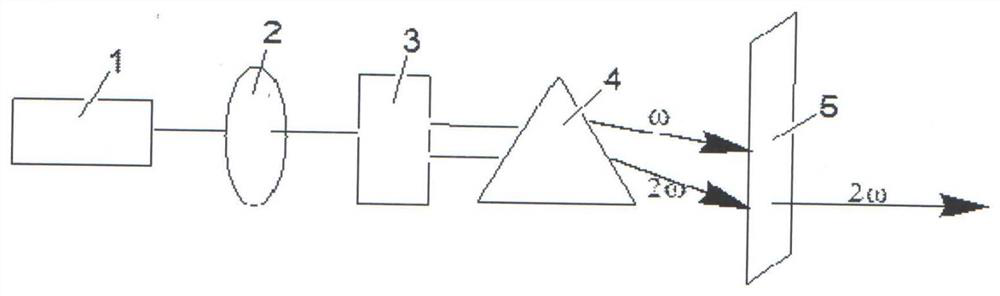
Compound rubidium boron nitrate, rubidium boron nitrate nonlinear optical crystal, and preparation method and application of compound rubidium boron nitrate nonlinear optical crystal
ActiveCN112919486ANovel Structural FeaturesGood optical performancePolycrystalline material growthFrom solid stateNonlinear optical crystalCrystal system
The invention provides a compound rubidium boron nitrate and a rubidium boron nitrate nonlinear optical crystal as well as a preparation method and application thereof, the chemical formula of the compound is Rb3B6O10NO3, the molecular weight is 543.28, and the compound is prepared by adopting a solid-phase synthesis method or a vacuum packaging method; and the chemical formula of the crystal compound is Rb3B6O10NO3, the molecular weight is 543.28, the crystal compound belongs to a trigonal system, the space group is P3221, the cell parameters are as follows: a = 8.3764 angstroms, b = 8.3764 angstroms, c = 15.6901angstroms, alpha = beta = 90 degrees, gamma = 120 degrees, the unit cell volume is 953.3 angstrom<3>, the frequency-doubled effect of the crystal is about 0.2 times of that of KH2PO4 (KDP), the ultraviolet cut-off edge is lower than 300 nm, the crystal is grown by adopting a vacuum packaging method or a cosolvent method, and the crystal has relatively excellent comprehensive properties. The crystal can be used as an ultraviolet nonlinear optical crystal in an all-solid-state laser.
Owner:XINJIANG TECHN INST OF PHYSICS & CHEM CHINESE ACAD OF SCI
Organic electroluminescence device and preparation method thereof
InactiveCN104659221ALower injection barrierImprove injection efficiencySolid-state devicesSemiconductor/solid-state device manufacturingRubidium compoundGlass transition
An organic electroluminescence device comprises an anode, a hole injection layer, a hole transport layer, a luminescent layer, an electron transport layer, an electron injection layer and a cathode which are stacked in sequence, wherein the electron injection layer consists of a rubidium compound doped layer and an electron transport material doped layer; the rubidium compound doped layer comprises a rubidium compound material and a metallic oxide material doped in the rubidium compound material; the rubidium compound material is at least one of rubidium carbonate, rubidium chloride, rubidium nitrate and rubidium sulfate; the metallic oxide material is at least one of molybdenum trioxide, tungsten trioxide and vanadium pentoxide; the electron transport material doped layer comprises an electron transport material and an organosilicon small molecule material doped in the electron transport material; the glass transition temperature of the electron transport material is 50 DEG C to 100 DEG C. The organic electroluminescence device is relatively high in luminescence efficiency. The invention further provides a preparation method of the organic electroluminescence device.
Owner:OCEANS KING LIGHTING SCI&TECH CO LTD +2
Organic light-emitting device and production method thereof
InactiveCN104638144AEasy injectionEasy transferSolid-state devicesSemiconductor/solid-state device manufacturingElectron injectionRubidium compound
The invention relates to an organic light-emitting device and a production method thereof. The organic light-emitting device is of a layered structure and comprises an anode conducting substrate, a hole injection layer, a hole transmission layer, a light-emitting layer, an electron transmission layer, an electron injection layer and a cathode layer which are stacked in sequence; the electron injection layer comprises a rubidium compound layer, a ternary doping layer and a rhenium compound layer; the rubidium compound layer is made of rubidium carbonate, rubidium chloride and rubidium nitrate or rubidium sulfate, the ternary doping layer is made of materials of low-work function metal, high-work function metal and metal sulfide. The rubidium compound layer in the electron injection layer is low in melting point and easy to evaporate and plate; work function is low due to existing of metal ions, electron injection barrier between the electron transmission layer and the injection layer can be lowered, and injection of electrons is benefited.
Owner:OCEANS KING LIGHTING SCI&TECH CO LTD +2
Compound rubidium boron nitrate and rubidium boron nitrate nonlinear optical crystal and preparation method and use
ActiveCN112919486BNovel Structural FeaturesGood optical performancePolycrystalline material growthFrom solid stateNonlinear optical crystalCrystal system
The invention provides a compound rubidium boronitrate and rubidium boronitrate nonlinear optical crystal, preparation method and application thereof, and the chemical formula of the compound is Rb 3 B 6 O 10 NO 3 , with a molecular weight of 543.28, prepared by solid-phase synthesis or vacuum encapsulation; the chemical formula of the crystalline compound is Rb 3 B 6 O 10 NO 3 , the molecular weight is 543.28, it belongs to the trigonal crystal system, and the space group is P 3 2 21, the unit cell parameters are a =8.3764Å, b =8.3764Å, c =15.6901Å, α = β =90°, γ =120°, the unit cell volume is 953.3Å 3 , the frequency doubling effect of the crystal is about KH 2 PO 4 (KDP) 0.2 times, the UV cut-off edge is lower than 300 nm, and the crystal is grown by vacuum encapsulation method or cosolvent method.
Owner:XINJIANG TECHN INST OF PHYSICS & CHEM CHINESE ACAD OF SCI
Organic electroluminescent device and preparation method thereof
InactiveCN104659243ALower injection barrierImprove injection efficiencySolid-state devicesSemiconductor/solid-state device manufacturingIron(III) sulfideRubidium compound
An organic electroluminescent device comprises an anode, a hole injection layer, a hole transport layer, a light emitting layer, an electron transport layer, an electron injection layer, and a cathode, which are stacked in sequence. The electron injection layer is composed of a rubidium compound doped layer and a ferric salt doped layer. The rubidium compound doped layer includes a rubidium compound material group and an electron transport material doped in the rubidium compound material. The rubidium compound material includes at least one selected from rubidium carbonate, rubidium chloride, rubidium nitrate, and rubidium sulfate. The electron transport material includes at least one selected from 4,7-diphenyl-1,10-phenanthroline, 2-(4'-tert-butylphenyl)-5-(4'-biphenyl)-1,3,4-oxadiazole, 8-hydroxyquinoline aluminum, and N-arylbenzimidazole. The ferric salt doped layer includes a ferric salt material and a bipolar organic transport material doped in the ferric salt material. The ferric salt material includes at least one selected from ferric chloride, ferric bromide and ferric sulfide.
Owner:OCEANS KING LIGHTING SCI&TECH CO LTD +2
Organic electroluminescence device and preparation method thereof
InactiveCN104659276AImprove the scattering effectIncrease transfer rateSolid-state devicesSemiconductor/solid-state device manufacturingRubidium compoundElectron
An organic electroluminescence device comprises an anode, a hole injection layer, a hole transporting layer, a luminous layer, an electron transporting layer, an electron injection layer and a cathode which are overlapped in sequence, wherein the electron injection layer is made of one or more rubidium compound materials, one or more metal oxide materials and zinc powder; the rubidium compound material(s) is / are one or more of rubidium carbonate, rubidium chloride, rubidium nitrate and rubidium sulfate; the metal oxide material(s) is / are one or more of molybdenum trioxide, tungsten trioxide and vanadic oxide. The light efficiency of the organic electroluminescence device is relatively high. The invention further provides a preparation method of the organic electroluminescence device.
Owner:OCEANS KING LIGHTING SCI&TECH CO LTD +2
Organic light-emitting device and production method thereof
InactiveCN104638140AGuaranteed performanceStable in natureSolid-state devicesSemiconductor/solid-state device manufacturingRubidium compoundElectron transmission
The invention relates to an organic light-emitting device and a production method thereof. The organic light-emitting device comprises an anode conducting substrate, a hole injection layer, a hole transmission layer, a light-emitting layer, an electron transmission layer, an electron injection layer and a cathode layer which are stacked in sequence; the electron injection layer comprises a rubidium compound doping layer and an iron salt layer; the rubidium compound doping layer is made of materials of a rubidium compound, low-work function metal and high-work function metal; the rubidium compound is made of materials of rubidium carbonate, rubidium chloride, rubidium nitrate or rubidium sulfate. The rubidium compound of the organic light-emitting device is low in melting point and easy to evaporate and plate; due to existing of metal ions, work function is low, an electronic injection barrier between the electron transmission layer and the injection layer can be reduced, and injection of the electron is benefited.
Owner:OCEANS KING LIGHTING SCI&TECH CO LTD +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com