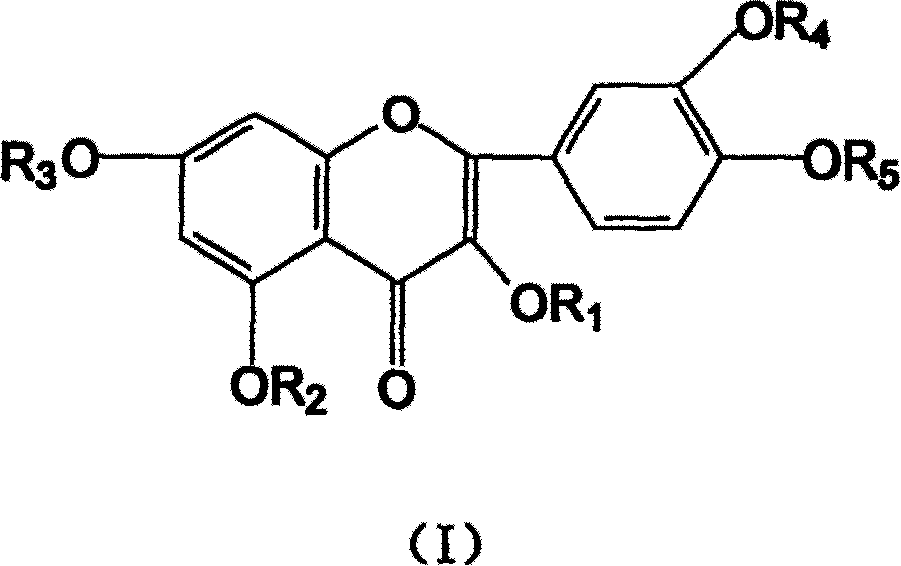
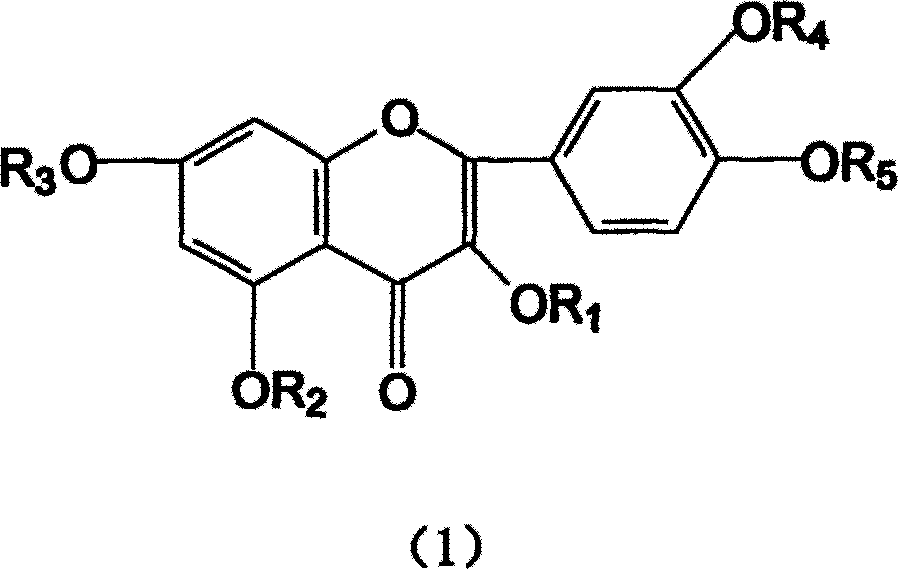
Ethoxyl quercetin derivative, method for preparation and use
A technology of quercetin and its compounds, applied in the field of quercetin derivatives and its preparation, achieving the effects of high yield, broad market development prospects, and simple preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] In a 500mL flask, add 4.0g NaOH and 250mL water, stir to dissolve. Add 60g of quercetin afterwards, stir to make it all dissolve. 32 mL of ethylene oxide was added dropwise with stirring, and the reaction was continued for another 30 hours under airtight conditions. The reaction solution was neutralized to neutral with 2mol / L hydrochloric acid, desalted with ion exchange resin, concentrated by heating under reduced pressure (70°C), and spray-dried to obtain 58.6g of tetrahydroxyethyl quercetin.
[0038] The chemical structure of the obtained product was identified by mass spectrometry and nuclear magnetic resonance.
[0039] The appearance of the product is light yellow powder, and the hydrochloric acid-magnesium powder reaction is positive, showing flavonoids.
[0040] Mass Spectrum (FAB-MS): m / z 478.2 [M] + , 434.1 (tetrahydroxyethyl quercetin lost a hydroxyethyl fragment).
[0041] nuclear magnetic resonance 17 CNMR (75MHz, DMSO-d 6 ), δppm: 146.7(C-2), 136.7(C...
Embodiment 2
[0043] In a 250mL flask, add 1.4gNaH, 200mL DMF, stir to dissolve. Then add 30g of quercetin and stir until dissolved. 20 mL of chloroethanol was added dropwise with stirring, and the reaction was continued for 24 hours. Neutralize the reaction liquid with 1mol / L sulfuric acid to neutral, concentrate under reduced pressure, desalt and remove DMF with a gel chromatography column, and dry under vacuum (20kPa) to obtain 26.3g of hydroxyethyl quercetin derivatives.
Embodiment 3
[0045] In a 250mL flask, add 10.2g KOH and 100mL water, stir to dissolve. Then add 15g of quercetin and stir until dissolved. 3 mL of ethylene oxide was added dropwise with stirring, and the reaction was continued for another 22 hours under airtight conditions. Use 2mol / L acetic acid to neutralize the reaction solution to neutrality, extract the water phase through n-butanol extraction, heat the n-butanol phase under reduced pressure (below 80°C) to recover n-butanol, and then vacuum-dry to obtain 13.2g of hydroxyl Ethyl quercetin derivatives.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com