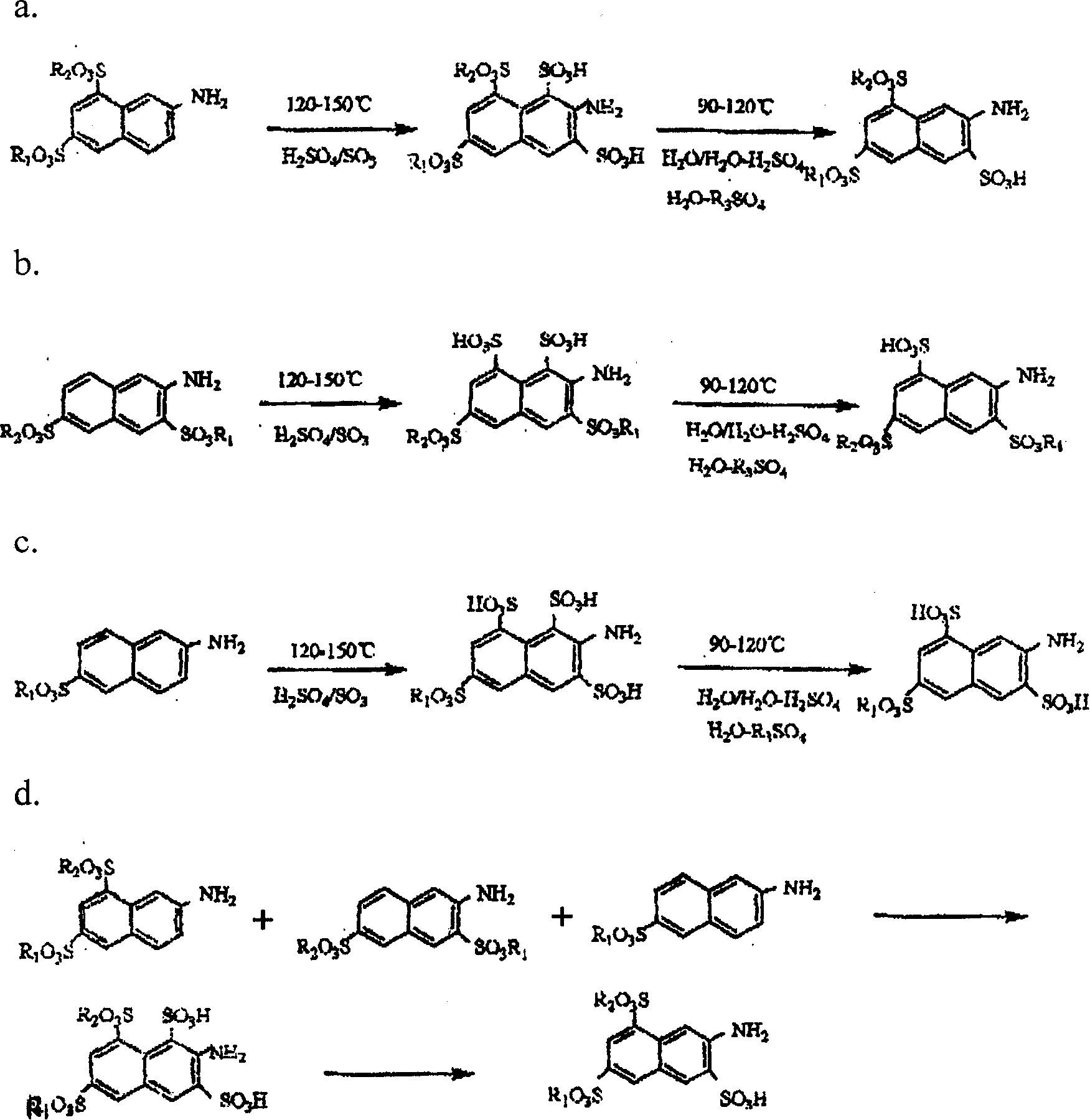
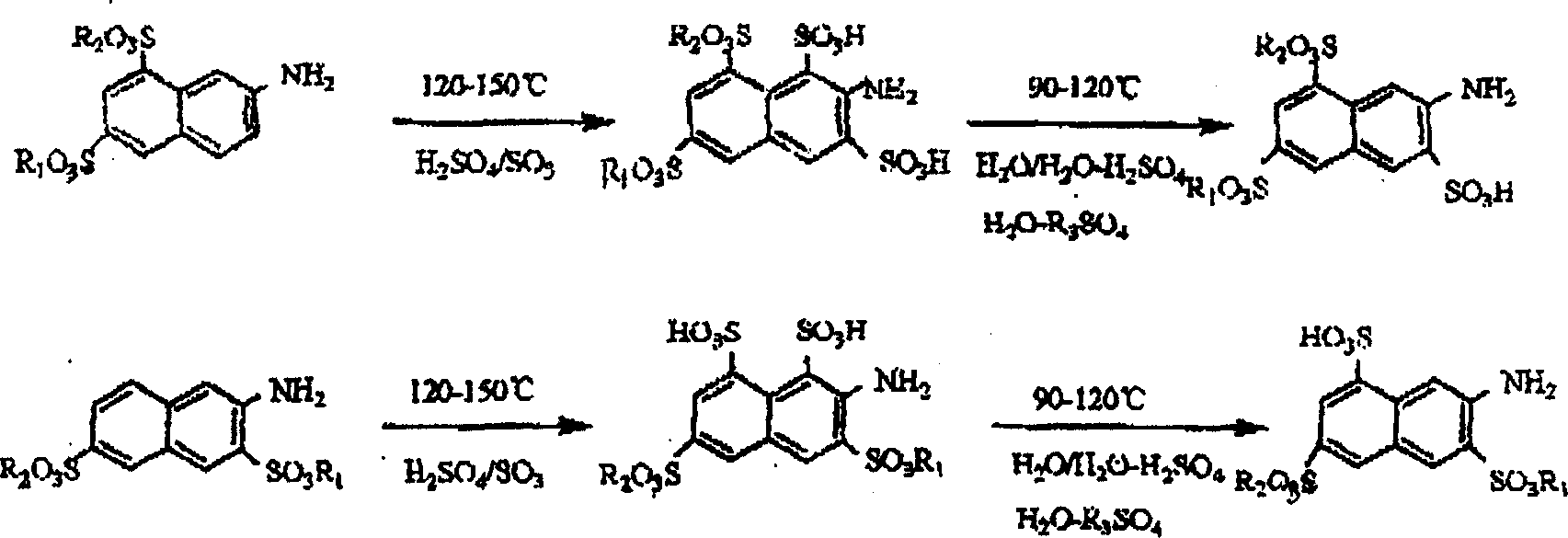
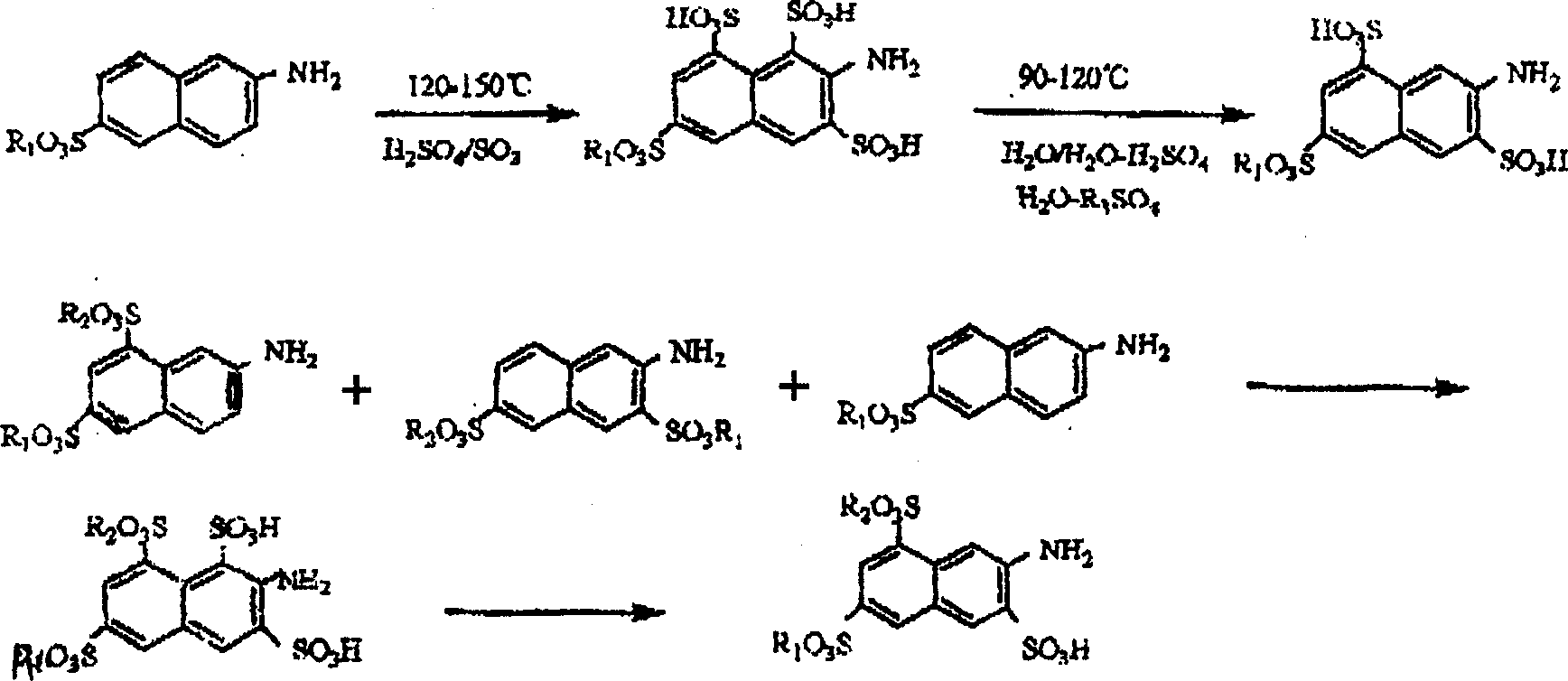
Prepn of 2-naphthylamine-3,6,8-trisulfonic acid
A technology of trisulfonic acid and naphthylamine, applied in chemical instruments and methods, azo dyes, organic dyes, etc., can solve the problems of preparation that have not been reported, and achieve the effects of easy availability of raw materials, wide application range, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Add 2200 parts (parts by weight, the same below) of 65% oleum into the sulfonation pot, and add 656 parts of dried amino G salt in batches under stirring. The feeding temperature does not exceed 70°C. After the addition, close the sulfonation pot, heat it to 125°C, and keep it at 125-140°C for 6 hours, with a pressure of 1-2 kg / cm2. Cool down to 100°C, and dilute the sulfonated compound into a dilution pot filled with 3000 parts of water. Dilution time is about 1-2 hours, the temperature after dilution is 110-120° C., the concentration of sulfuric acid is in the range of 40-50%, and it is incubated under this condition for 1-2 hours. Cool down to 25-35°C, filter to obtain 770 parts of amino acid K. The yield was 92.88%, and the purity by liquid chromatography analysis was 99.8%.
Embodiment 2
[0020] Add 2200 parts (parts by weight, the same below) of 65% oleum into the sulfonation pot, and add 656 parts of dried amino R salt in batches under stirring. The feeding temperature should not exceed 70°C. After the addition, the sulfonation pot should be closed and heated to 125°C for 6 hours, with a pressure of 1-2 kg / cm2. Cool down to 100°C, and dilute the sulfonated compound into a dilution pot filled with 3000 parts of water. The dilution time is about 2-3 hours, the temperature after dilution is 110-120° C., the concentration of sulfuric acid is in the range of 35-50%, and the temperature is kept for 1-2 hours under this condition. Cool down to 25-35°C, filter to obtain 770 parts of amino acid K. Yield 88%, liquid chromatography analysis purity 99.64%
Embodiment 3
[0022] Add 2300 parts (parts by weight, hereinafter the same) 65% oleum into the sulfonation pot, add the mixture formed by mixing the dried amino G salt, amino R salt and Bronic acid in batches with a stirring rate of 710.5% Among them, 576 parts of amino G salt; 108 parts of amino R salt; 26.5 parts of bronnic acid. The feeding temperature does not exceed 70°C. After the addition, close the sulfonation pot, heat it to 125°C, and keep it at 125-140°C for 6 hours, with a pressure of 1-2 kg / cm2. Cool down to 100°C, and dilute the sulfonated compound into a dilution pot filled with 3000 parts of water. The dilution time is about 1-2 hours. Cool down to 25-35°C, filter to obtain 871.7 parts of amino acid K. The yield was 95.8%, and the purity by liquid chromatography analysis was 99.63%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com