Crystalline form iv of agomelatine, a process for its preparation and pharmaceutical compositions containing it
A technology of composition and crystal form, applied in the field of new crystal form IV of N-[2-ethyl]acetamide, can solve problems such as no specific description
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
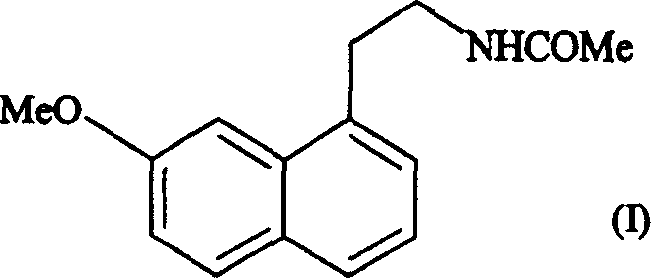
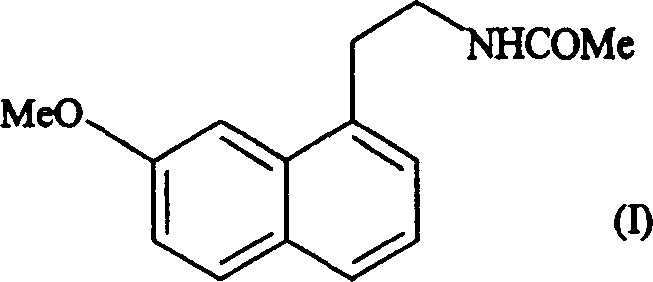
[0018] Example 1: Form IV of N-[2-(7-methoxy-1-naphthyl)ethyl]acetamide
[0019] 2θ angle (°)
Embodiment 2
[0020] Embodiment 2: pharmaceutical composition
[0021] Prepare a prescription for 1000 tablets, each containing a 25mg dose:
[0022] The compound of Example 1……………………………… 25g
[0023] Lactose monohydrate…………………………………………… 62g
[0024] Magnesium stearate…………………………………………… 1.3g
[0025] Corn starch…………………………………………………………… 26g
[0026] Maltodextrin mixture……………………………………9g
[0027] Anhydrous colloidal silicon dioxide……………………………………… 0.3g
[0028] Sodium Starch Glycolate Type A………………………………… 4g
[0029] Stearic acid……………………………………………………… 2.6g
Embodiment 3
[0030] Embodiment 3: pharmaceutical composition
[0031] Prepare a prescription for 1000 tablets, each containing a 25mg dose:
[0032] The compound of Example 1……………………………… 25g
[0033] Lactose monohydrate…………………………………………… 62g
[0034] Magnesium stearate…………………………………………… 1.3g
[0035] Povidone……………………………………………………9g
[0036] Anhydrous colloidal silicon dioxide……………………………………… 0.3g
[0037] Sodium carboxymethylcellulose………………………………………………………………………………………………………30g
[0038] Stearic acid …………………………………………………… 2.6g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com