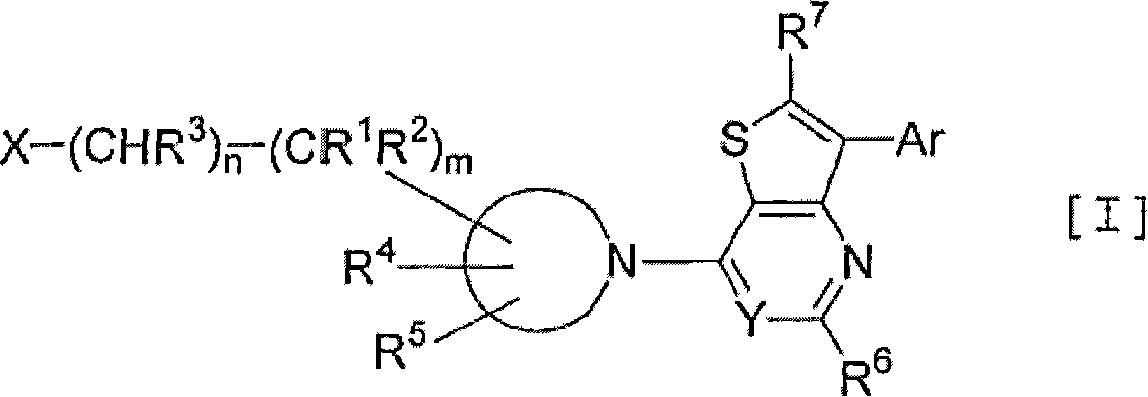
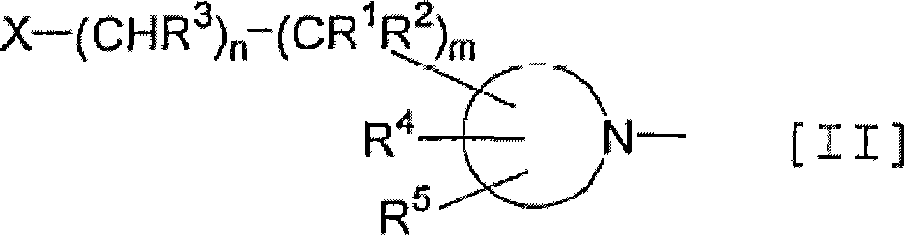
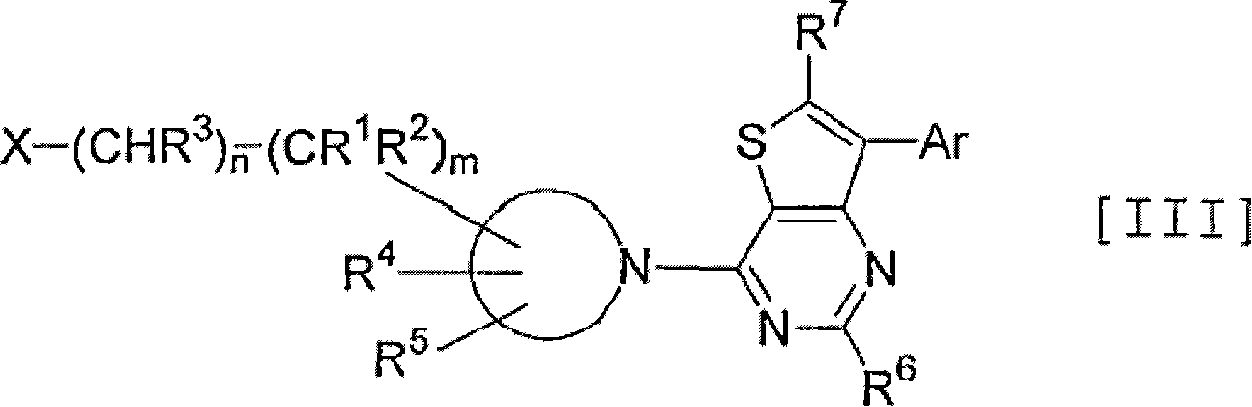
Thienopyrimidine and thienopyridine derivatives substituted with cyclic amino group
A cyclic amino and pyrimidine technology, which can be used in drug combinations, digestive system, endocrine system diseases, etc., and can solve problems such as undisclosed compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0132] Synthesis of {1-[7-(4-bromo-2,6-dimethyl-phenyl)-2,6-dimethyl-thieno[3,2-d]pyrimidin-4-yl]-piperidine -4-yl}-methanol hydrochloride (compound 1-004)
[0133]
[0134] (1) 7-(4-bromo-2,6-dimethyl-phenyl)-4-chloro-2,6-dimethyl-thieno[3,2-d]pyrimidine (500mg), piperidine - A mixture of 4-ylmethanol (226 mg), N,N-diisopropylethylamine (253 mg) in ethanol (1.5 mL) was heated at reflux for 1 day. After the reaction mixture was cooled to room temperature, it was poured into saturated aqueous sodium bicarbonate solution, and then extracted with EtOAc. The organic layer was washed with brine, dried over anhydrous sodium sulfate and filtered. The filtrate was concentrated under reduced pressure and purified by silica gel column chromatography (silica gel: Wako Gel (C200), eluent: hexane / EtOAc=3:1) to give {1-[7- (4-bromo-2,6-dimethyl-phenyl)-2,6-dimethyl-thieno[3,2-d]pyrimidin-4-yl]-piperidin-4-yl}-methanol (568 mg).
[0135] (2) Under ice cooling, in {1-[7-(4-bromo-2,6-d...
Embodiment 2
[0138] {1-[7-(4-bromo-2,6-dimethyl-phenyl)-2,6-dimethyl-thieno[3,2-d]pyrimidin-4-yl]-piperidine- 4-yl}-acetic acid
[0139]
[0140] (1) {1-[7-(4-bromo-2,6-dimethyl-phenyl)-2,6-dimethyl-thieno[3,2-d]pyrimidin-4-yl]- A mixture of piperidin-4-yl}-acetonitrile (350 mg) and KOH (492 mg) in EtOH (1.5 mL) and water (1.0 mL) was heated in a sealed tube at 105°C for 3 hours. After the reaction mixture was concentrated under reduced pressure, 5% KHSO was added 4 aqueous solution with CHCl 3 extraction. The organic layer was washed with brine and washed with anhydrous Na 2 SO 4 Dry and concentrate under reduced pressure. The residue was purified by silica gel column chromatography (silica gel: Wako Gel (C200), eluent: CHCl 3 / methanol=20:1) to obtain the title compound (164 mg).
[0141] Table 1 lists the compounds obtained in Example 2 and the compounds obtained according to the similar steps described in Example 2.
Embodiment 3
[0143] 2-{1-[7-(4-bromo-2,6-dimethyl-phenyl)-2,6-dimethyl-thieno[3,2-d]pyrimidin-4-yl]-piper Pyridine-4-yl}-acetamide
[0144]
[0145] {1-[7-(4-bromo-2,6-dimethyl-phenyl)-2,6-dimethyl-thieno[3,2-d]pyrimidin-4-yl]-piperidine- 4-yl}-acetonitrile hydrochloride (30mg) was dissolved in CH 2 SO 4 (0.5 mL), and the solution was stirred at room temperature for 20 hours. After adding ice, use NaOH aqueous solution and NaHCO 3 Aqueous solution made the reaction mixture basic (pH 7). The mixture was extracted with EtOAc and the organic layer was washed with brine, washed with anhydrous Na 2 SO 4 Dry and concentrate under reduced pressure. The residue was purified by silica gel column chromatography (silica gel: Wako Gel (C200), eluent: EtOAc) to obtain the title compound (20 mg) as white crystals.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com