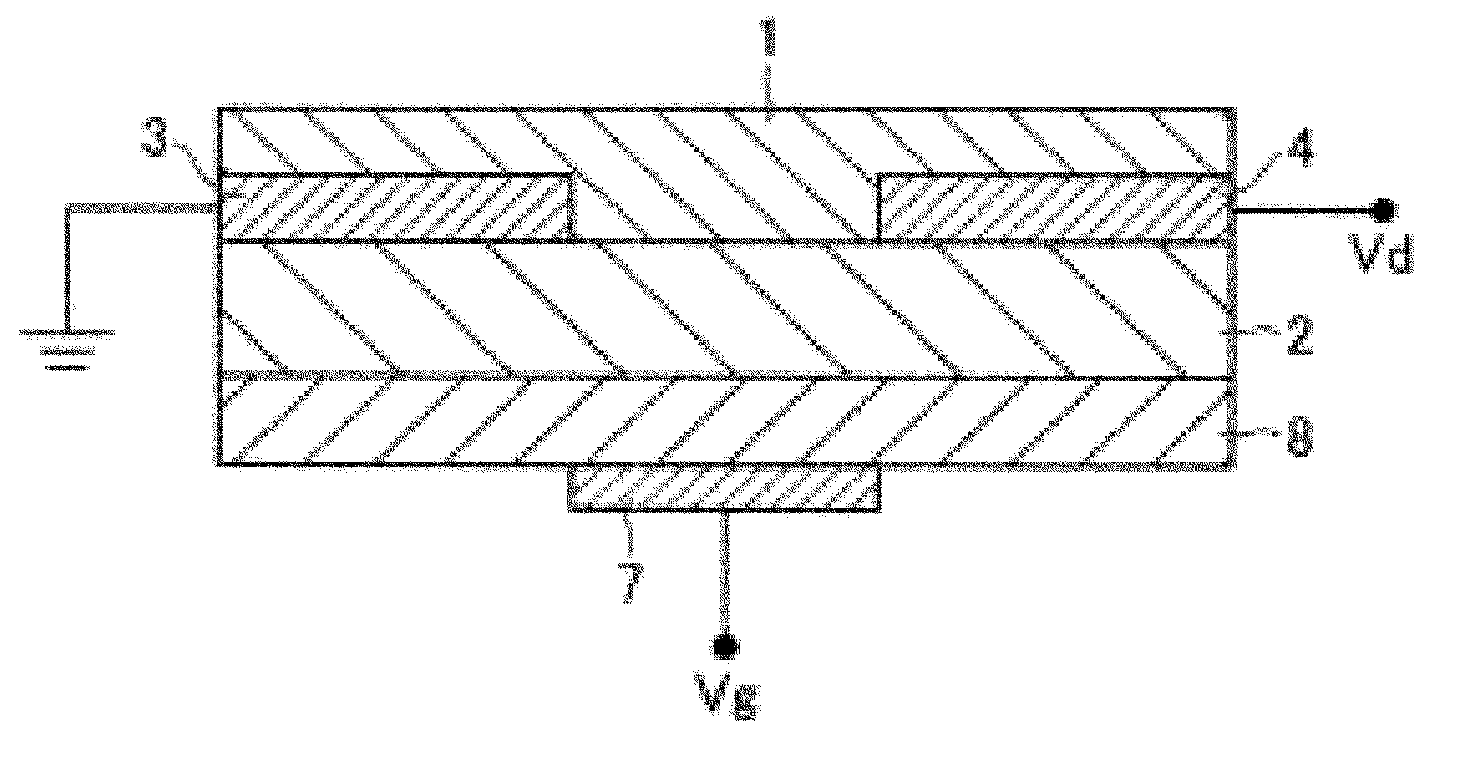
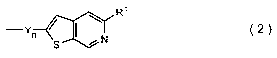Thienopyridine derivative, method for producing same and organic semiconductor device using same
A technology of organic semiconductors and derivatives, applied in the fields of semiconductor devices, semiconductor/solid-state device manufacturing, electric solid-state devices, etc., can solve the problem that the characteristics may not be sufficient
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0106] (Synthesis Example 1) [Synthesis of 5-phenylsulfonylthieno[2,3-c]pyridine represented by formula (6a)]
[0107] In a three-necked flask with an inner volume of 300ml equipped with a thermometer, a magnetic stirrer and a dropping funnel, benzenesulfonylcarbonitrile (3.77g, 22.6mmol) and isobutyl chloroformate (3.08g, 22.6mmol) were xylene (100ml) and stirred under reflux. To this mixture was added dropwise a solution of 3-methylthiophene-2-carbaldehyde-N-phenylimine (3.03 g, 15 mmol) in xylene (50 ml) over 30 minutes. After completion of the dropwise addition, the reaction mixture was heated under reflux for 1 hour, and after standing to cool to room temperature, the solvent was distilled off to obtain a crude product. These were purified by silica gel column chromatography to obtain 2.79 g of 5-benzenesulfonylthieno[2,3-c]pyridine (10.1 mmol, yield 67%). The chemical reaction formula is shown below.
[0108]
[0109] The NMR data of 5-benzenesulfonylthieno[2,3-c]p...
Synthetic example 2
[0111] (Synthesis Example 2) [Synthesis of 2-bromo-5-benzenesulfonylthieno[2,3-c]pyridine represented by formula (3a)]
[0112] Add 5-benzenesulfonylthieno[2,3-c]pyridine (2.37g, 8.6mmol), N-bromosuccinimide ( 4.60 g, 25.8 mmol) and 30 ml of acetonitrile, heated and stirred at 70° C. for 6 hours. After the reaction liquid was left to cool to room temperature, the solvent was distilled off, and 100 ml of ethyl acetate and 100 ml of water were added to the obtained residue. The organic layer was separated from the water layer with a separatory funnel, and the water layer was extracted twice with 50 ml of ethyl acetate. It was mixed with the previously separated organic layer, and after washing twice with 100 ml of water, the organic layer was dried over anhydrous magnesium sulfate. The crude product obtained by distilling off the solvent with toluene and ethyl acetate was purified by recrystallization to obtain 2.49 g of 2-bromo-5-benzenesulfonylthieno[2,3-c]pyridine (7.0 mmol...
Synthetic example 3-1
[0116] (Synthesis Example 3-1) [Synthesis of 2-bromo-5-methoxythieno[2,3-c]pyridine represented by formula (3b)]
[0117] Add 2-bromo-5-benzenesulfonylthieno[2,3-c]pyridine (1.00g, 2.8mmol), 28 % methanol solution (1.62g, 8.4mmol) and 20ml of tetrahydrofuran, heated and stirred at 60°C for 6 hours. The reaction solution was left to cool to room temperature, and after adding 1 ml of acetic acid, the solvent was distilled off, and 50 ml of ethyl acetate and 50 ml of water were added to the obtained residue. The organic phase was separated from the aqueous phase with a separatory funnel, and the organic phase was dried over anhydrous magnesium sulfate. The crude product obtained by distilling off the solvent was purified by silica gel column chromatography to obtain 0.635 g of 2-bromo-5-methoxythieno[2,3-c]pyridine (2.6 mmol, yield: 93%). The chemical reaction formula is shown below.
[0118]
[0119] The NMR data of 2-bromo-5-methoxythieno[2,3-c]pyridine represented by the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com