Insulin liquid formulations for nose administration
A liquid preparation, insulin technology, applied in the directions of drug combination, drug delivery, pharmaceutical formulation, etc., can solve the problems of irreversible damage of nasal cilia, no breakthrough of insulin, damage of nasal mucosa, etc., to avoid adverse reactions, prolong residence time, and effect time. quick effect
Inactive Publication Date: 2007-02-28
SHANGHAI INST OF PHARMA IND CO LTD
View PDF3 Cites 11 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
[0005] Chinese patent [Application No.] 01801146.2, nasal inhalation preparation of insulin, discloses an insulin-containing preparation using porous spherical calcium carbonate as a carrier, wherein the porous spherical calcium carbonate is columnar or needle-like microcrystals or their parallel contiguous crystals Aggregates; the defect of the technology disclosed in this patent is that it only solves the difficulty of long-term retention in the nasal cavity after the adsorption of insulin and the carrier, but does not break through the barrier of insulin absorbed into the body through the nasal mucosa;
[0006] Chinese patent [Application No.] 02125051.0, composition for oral and nasal administration, dry powder inhaler and its preparation method, the invention takes natural milk and natural fragrance as the main line, proposes carrier-free dry powder inhaler composition, inhaler and its Preparation method; the defects in the technology disclosed in this patent are as using natural milk such as milk directly through vacuum spray-drying (or freeze-drying) to prepare the carrier, which is likely to cause protein allergy when inhaled and administered, and insulin itself has no peculiar smell. no flavoring required;
However, sodium glycinate, as an absorption accelerator, has irreversible damage to nasal cilia, and long-term use will cause damage to nasal mucosa
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
[0034] insulin
Embodiment 2
[0036] insulin
Embodiment 3
[0038] insulin
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Login to View More
Abstract
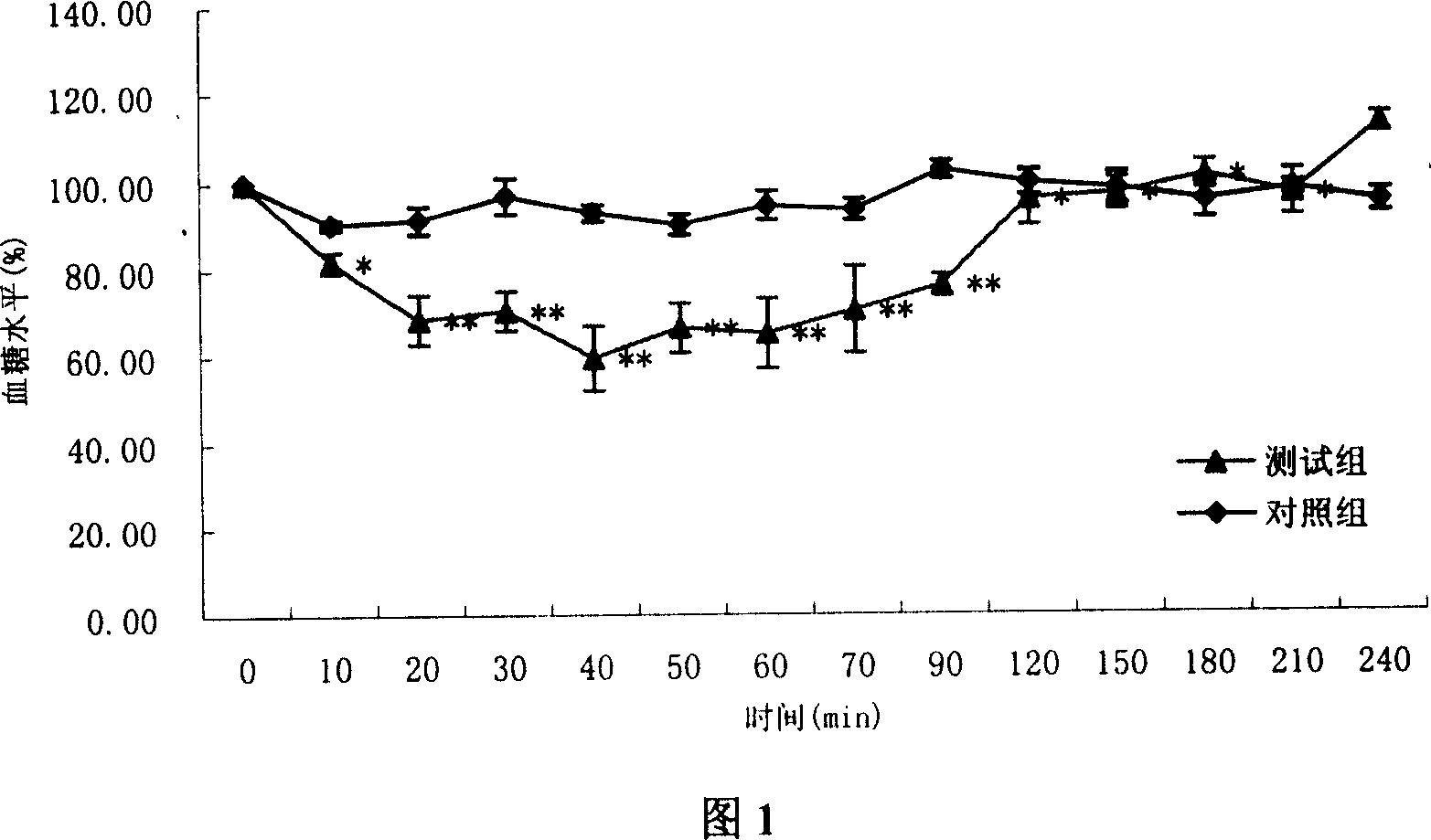
The invention discloses insulin providing liquid agent through nose, which is characterized by the following: avoiding side-effect due to injecting drug, increasing drug adsorbing area because the nose possesses bigger nasal area (150-180cm<2>) with large scale of microvillus and thinner nasal mucosa (2-4mm), adsorbing drug rapidly due to abundant capillary and capillary lymphangiolog.
Description
technical field [0001] The invention relates to an insulin preparation, in particular to a liquid preparation for nasal administration of insulin. Background technique [0002] With the development of biotechnology and genetic engineering, more and more polypeptide and protein drugs are used in clinical treatment, and most polypeptide drugs are biologically active and unstable to heat and enzymes, so they are often given by injection. medicine. However, long-term injection administration brings great pain to patients, and requires special injection techniques, which makes it difficult for patients to administer medicines independently, resulting in high finished products and high medical expenses. Therefore, domestic and foreign pharmaceutical workers have been committed to the research of non-injection routes of polypeptide drugs, such as nasal cavity, rectal, oral, lung, eye, transdermal absorption and so on. Among the above routes of administration, nasal administration...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): A61K38/28A61K9/00A61P3/10
Inventor 金方闻聪
Owner SHANGHAI INST OF PHARMA IND CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com