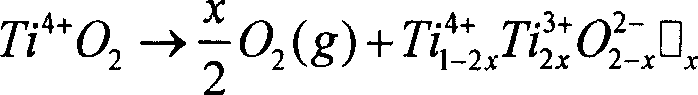Synthesis method of photochromic titanium dioxide sol
A technology of titanium dioxide and synthesis methods, applied in chemical instruments and methods, color-changing fluorescent materials, etc., can solve problems that limit the practical application of color-changing materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] At room temperature, under the condition of magnetic stirring, titanium tetrachloride (TiCl 4 ) was slowly added dropwise to the ethanol solution dissolved in triethanolamine (TEA). The reaction is violent, a large amount of heat is released, and a large amount of acid mist is formed, forming a pasty complex. TiCl 4 The substance ratio with TEA is 1:2, ethanol is used as solvent, and its volume is 3 times that of triethanolamine. After the complex was kept at about 30°C for a period of time, deionized water was added to dissolve it to form a saturated solution. Under the condition of magnetic stirring, 5 ml of the prepared saturated solution was added dropwise to 100 ml of AMP95 aqueous solution with a mass percentage concentration of 1.5% to obtain nano anatase titanium dioxide sol. The beaker containing the sol was placed on the windowsill, and it was found that the titanium dioxide sol on the side of the beaker near the window turned purple-black.
Embodiment 2
[0018] At room temperature, under the condition of magnetic stirring, titanium tetrachloride was slowly added dropwise to the ethanol solution dissolved with triethanolamine. The reaction is violent, a large amount of heat is released, and a large amount of acid mist is formed, forming a pasty complex. TiCl 4 The substance ratio with TEA is 1:2, ethanol is used as solvent, and its volume is 3 times that of triethanolamine. After the complex is kept at about 20°C for a period of time, it is dissolved in deionized water to form a saturated solution. Under the condition of magnetic stirring, 2ml of the prepared saturated solution was added dropwise to the AMP95 aqueous solution with a mass percentage concentration of 2% and 100ml to obtain nano-anatase titanium dioxide sol. The beaker containing the sol was placed on the windowsill, and it was found that the titanium dioxide sol on the side of the beaker near the window turned purple-black.
Embodiment 3
[0020] At room temperature, under the condition of magnetic stirring, titanium tetrachloride was slowly added dropwise to the ethanol solution dissolved with triethanolamine. The reaction is violent, a large amount of heat is released, and a large amount of acid mist is formed, forming a pasty complex. TiCl 4 The substance ratio with TEA is 1:2, ethanol is used as solvent, and its volume is 3 times that of triethanolamine. After the complex is kept at about 60°C for a period of time, it is dissolved in deionized water to form a saturated solution. Under the condition of magnetic stirring, 10 ml of the prepared saturated solution was added dropwise to 100 ml of AMP95 aqueous solution with a mass percentage concentration of 0.5% to obtain nano anatase titanium dioxide sol. The beaker containing the sol was placed on the windowsill, and it was found that the titanium dioxide sol on the side of the beaker near the window turned purple-black.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com