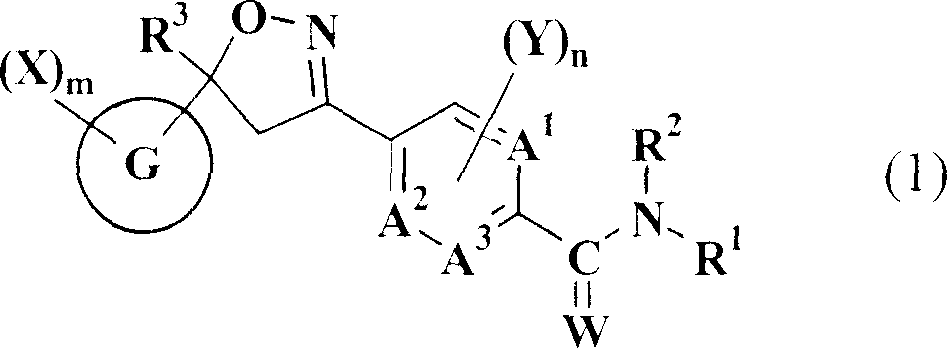
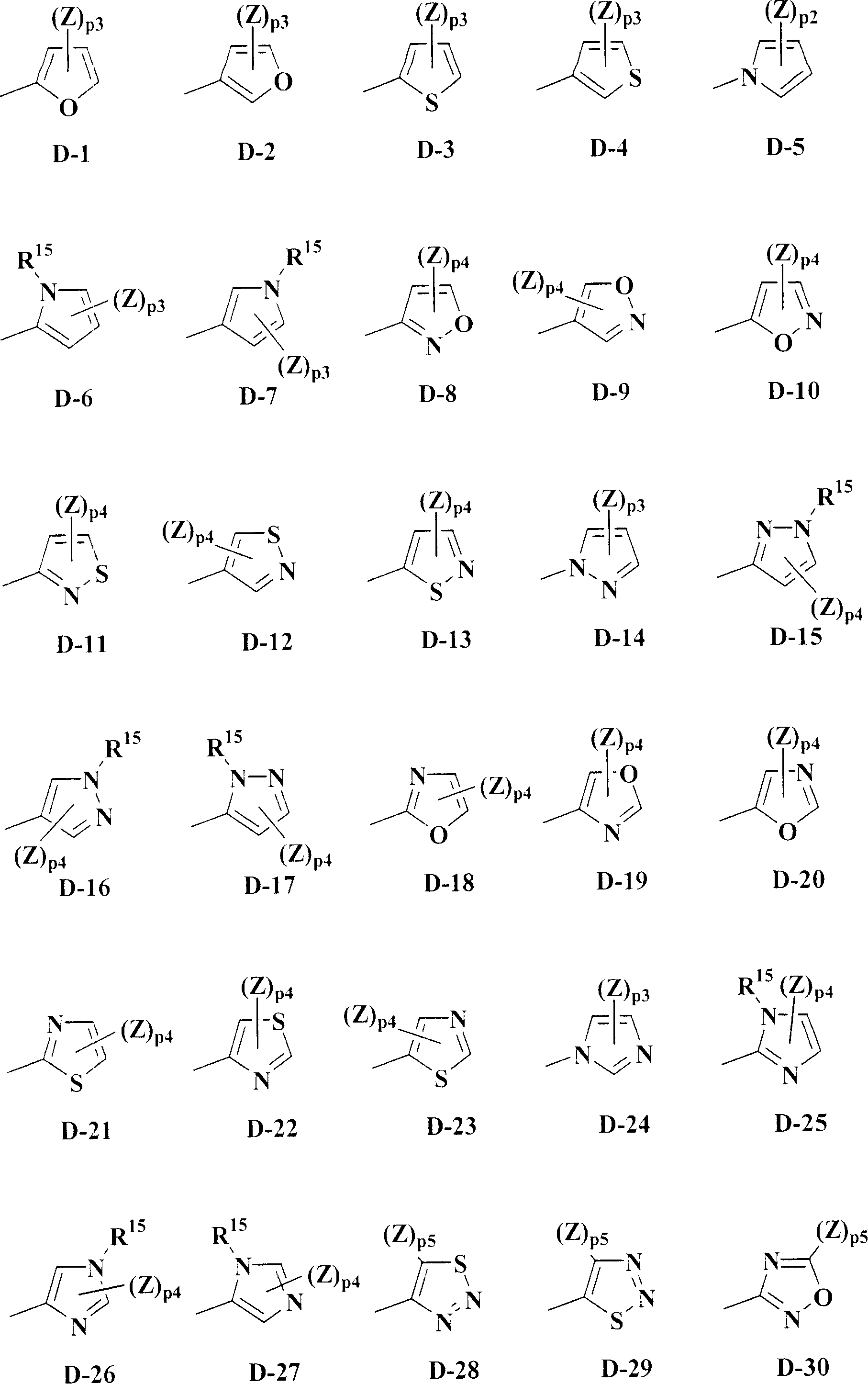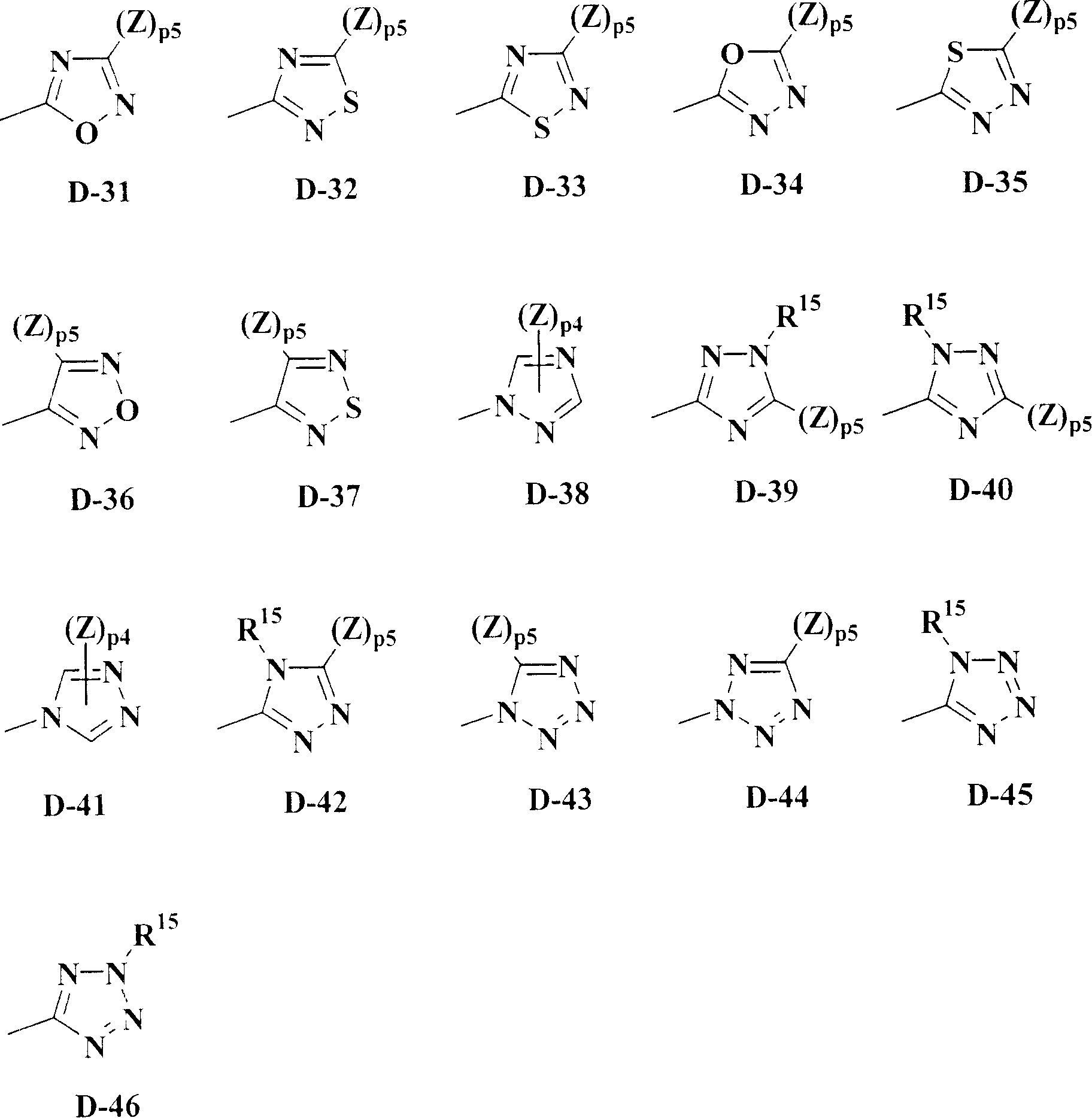Isoxazoline-substituted benzamide compound and noxious organism control agent
A technology of benzamides and compounds, applied in the field of pest control agents, can solve the problems of undisclosed and unknown usefulness of pest control agents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0828] Hereinafter, as examples, the synthesis examples and test examples of the present invention will be described in more detail, but the present invention is not limited to these.
Synthetic example 1
[0831] Production of the compound of the present invention using L-COS (a parallel liquid layer synthesis device manufactured by MRITEX).
[0832] Weigh n-propylamine, isopropylamine, sec-butylamine, tert-butylamine, n-pentylamine, 2,2-dimethylpropylamine, n-hexylamine, cyclohexylamine, and benzylamine in 15 L-COS reaction tubes equipped with a stir bar , 4-Trifluoromethylbenzylamine, 1-phenylethylamine, 2-phenylethylamine, 2-(4-phenoxyphenyl)ethylamine, 3-phenylpropylamine and trans-2-phenyl The cyclopropylamine is 1.5 mmol each, covered with a lid and set in the L-COS reaction tank. Stir at room temperature, add 5ml 4-[5-(3,4-dichlorophenyl)-5-trifluoromethyl-4,5-dihydroiso_oxazol-3-yl]benzene to each reaction tube N,N-dimethylformamide-chloroform (1:3) solution (0.2mmol / ml) of formic acid, 1ml 4-(dimethylamino)pyridine in chloroform solution (0.25mmol / ml), and then inject sequentially 1.5 ml of 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride in chloroform (1.0 mmol...
Synthetic example 2
[0849] Production of the compound of the present invention using L-COS (Parallel Liquid Layer Synthesis Apparatus from MRITEX).
[0850] Paste the numbers 1-10 on the 10 L-COS reaction tubes equipped with a stir bar, weigh 1.0mmol 3-chloro-4-fluorophenylboronic acid in the No.1 and No.6 reaction tubes, Weigh 1.0mmol 3,5-bis(trifluoromethyl)phenylboronic acid in the reaction tubes of .2 and No.7, and weigh 1.0mmol 3-trifluoromethylbenzene in the reaction tubes of No.3 and No.8 Boronic acid, weigh 1.0 mmol 3-trifluoromethoxyphenylboronic acid in No.4 and No.9 reaction tubes and weigh 1.0 mmol 2-naphthylboronic acid in No.5 and No.10 reaction tubes, then , Add 0.05mmol dichlorobis(triphenylphosphine)palladium(II) to each reaction tube, seal it with nitrogen, cover it, and set it in the L-COS reaction tank. Stir at room temperature, and inject 3ml of 2-bromo-3,3,3-trifluoropropane in 1,2-dimethoxyethane solution (0.5mmol / ml) and 1.5ml of water into each reaction tube. Then, 1 ml of a ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com