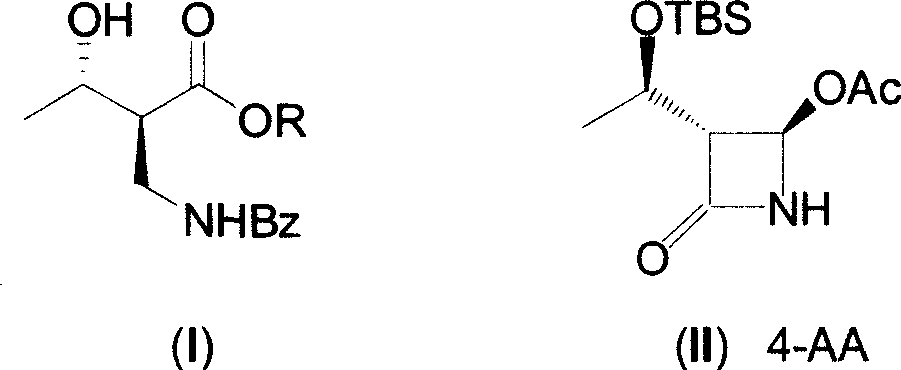Synthesis of (2S,3S)-2-benzoyl aminometh-3-hydroxy-butyrate ester series compound by asymmetric yeast cell
A technology for benzamidomethyl and compound, which is applied in the field of biological asymmetric synthesis of -2-benzamidomethyl-3-hydroxybutyrate, can solve the problems of restricting industrial production, long synthesis route and total yield. problems such as low rate, to achieve the effect of easy realization of reaction conditions, low cost and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0020] The preparation method of the present invention is further described in detail below by way of examples.
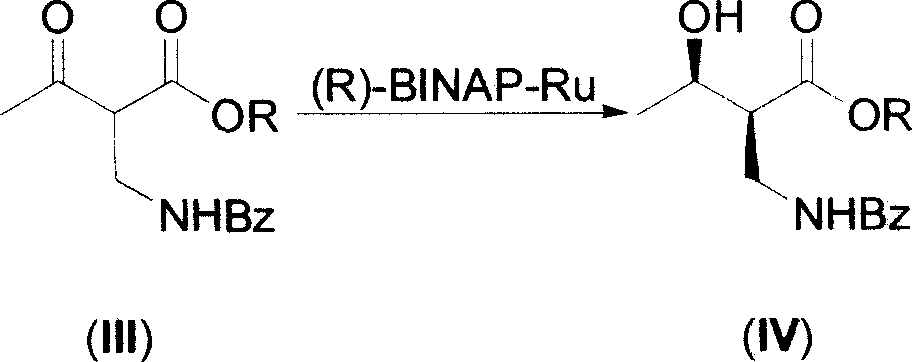
[0021] 1. (2S, 3S)-2-benzoylaminomethyl-3-hydroxybutyric acid ethyl ester is the compound of formula (I), (2R, 3S)-2-benzoylaminomethyl-3-hydroxybutyrate Ethyl acid ester is the preparation of formula (V) compound (R=Et):
[0022] Add tap water (2500ml), sucrose (600g), baker's yeast (100g, Angel Yeast, purchased in a supermarket) successively in a 5-liter three-necked round-bottomed flask equipped with a bubbler and a thermometer, and stir the mixture slightly (120r / min) .
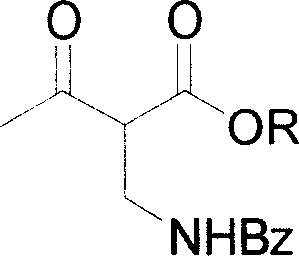
[0023] After 1 hr, make the generated CO 2 Gas escapes at a rate of about 1 to 2 bubbles / second. Then 2-benzamidomethyl-3-carbonylbutyrate (III, 35 g) was added thereto, and the mixture was stirred at 33˜36° C. for 24 hours.
[0024] Sucrose (150 g) was dissolved in 500 ml of tap water and added to the reaction mixture, stirring was continued at 33-36° C., and the reaction was monitored by...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com