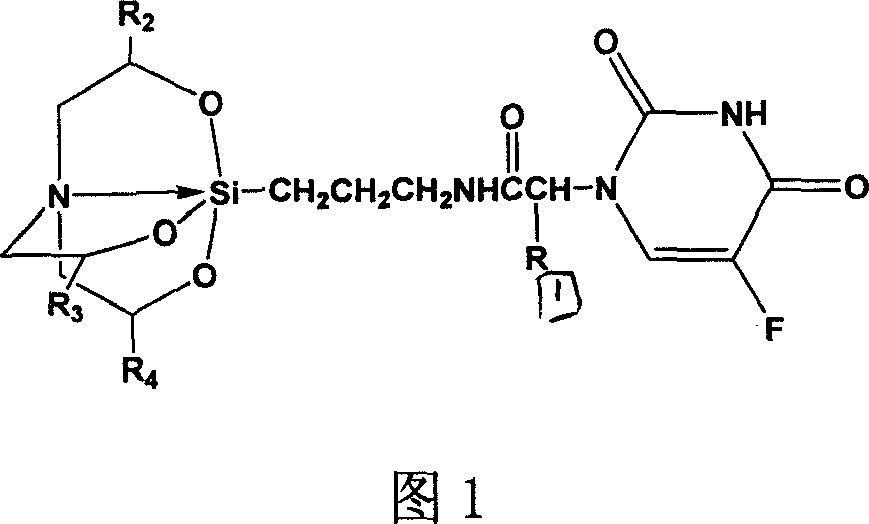
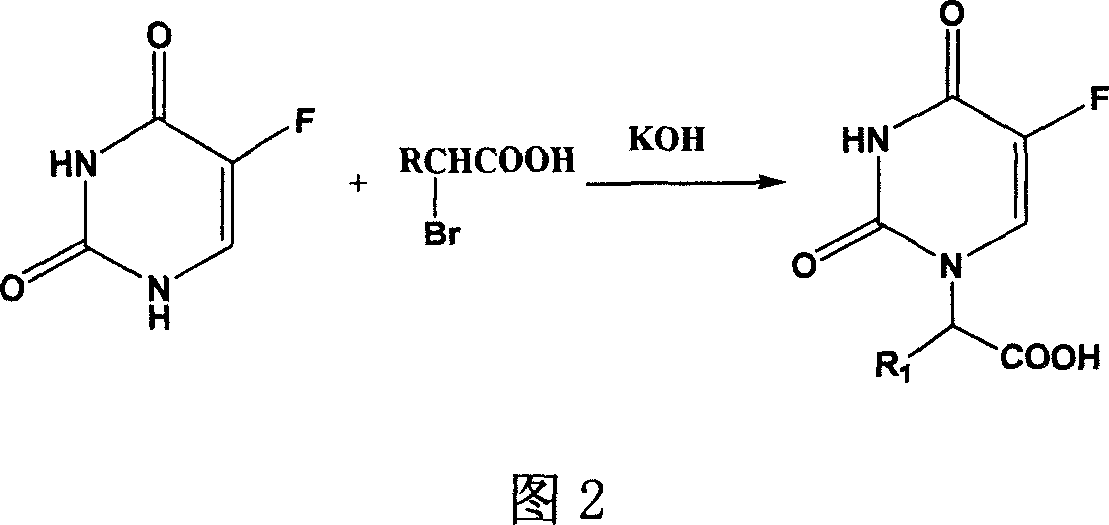
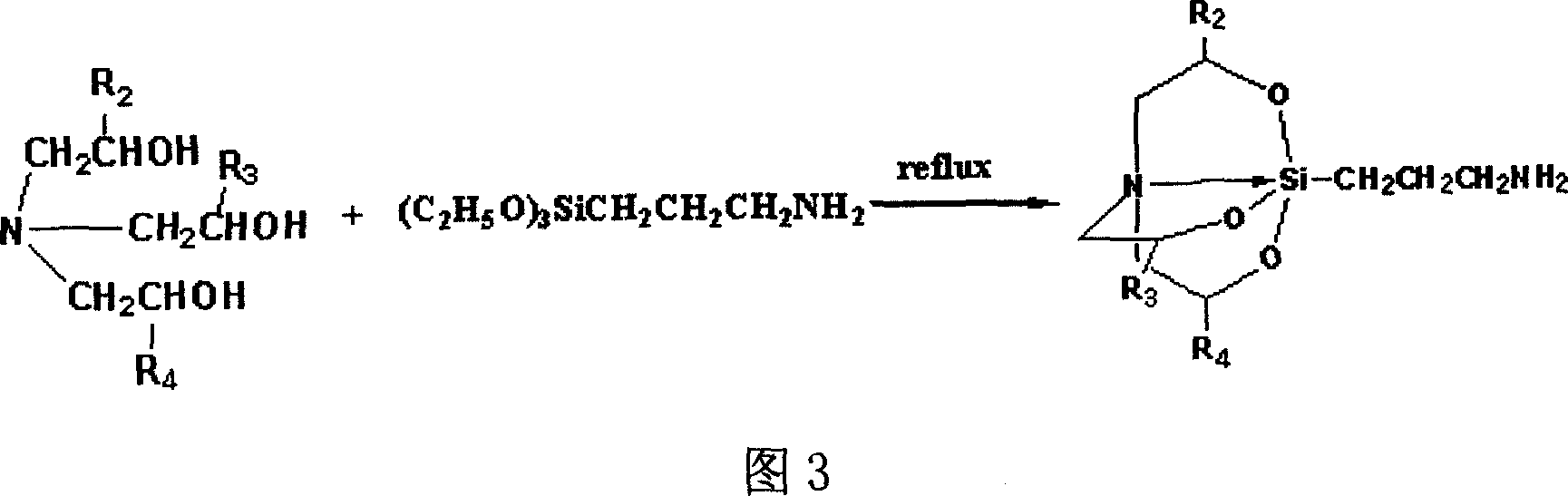
Preparation of 3,7,10-tri-substuent hetero nitrogen silicon tricyclic and 5-Fu combined substance and its use in pharmacy
A technology of three substitutions and conjugates, which is applied in the fields of compounds of group 4/14 elements of the periodic table, active ingredients of silicon compounds, antineoplastic drugs, etc. Low fat and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment 1
[0034] γ-[N-[1-oxo-2-[1-(5-fluoro-uracilyl)]ethyl]amino]azasilatricyclo(1)
[0035] 0.5g (2.66mmol) compound A (where R 1 hydrogen) and DCC 1.0g and p-nitrophenol 0.4g (2.88mmol) were added to dry 15ml DMF, heated to 40°C, reacted for 1h, added compound B (where R 2 , R 3 , R 4 hydrogen) 0.62g (2.66mmol), reacted for more than 24 hours, filtered to remove the urea compound generated, the filtrate was concentrated, the residue was purified by silica gel column chromatography [chloroform: methanol = 20: 1], and acetone: petroleum ether = 1:4 recrystallization, 0.29g white solid was obtained, yield: 27.1%, m.p.168-170℃. 1 H-NMR (CDCl 3 )δ: 10.64 (s, 1H, 6-fluorouracil), 7.83 (d, 1H, 3-fluorouracil), 7.28 (s, 1H, -CONH), 4.39 (s, 2H, -NCH 2 CO), 3.67(t, 6H, -Si(OCH 2 -) 3 ), 3.12(q, 2H, -CONHCH 2 CH 2 ), 2.82(t, 6H, -N(CH 2 ) 3 ), 1.52 (m, 2H, -CH 2 CH 2 CH 2 ), 0.25 (m, 2H, -CH 2 Si).ESI-MS(70V): 403.5[M+H] +.Anal.Calcd for C 15 h 23 N 4 o 6 FSi*0.25H 2 O(40...
specific Embodiment 2
[0036] 3,7,10-Trimethyl-γ-[N-[1-oxo-2-[1-(5-fluoro-uracilyl)]ethyl]amino]azasilatricyclo(2)
[0037] According to the preparation method of compound (1), drop into 0.5g (2.66mmol) compound A (wherein R 1 hydrogen) and DCC 1.0g and p-nitrophenol 0.4g (2.88mmol) were added to dry 15ml DMF, heated to 40°C, reacted for 1h, added compound B (where R 2 , R 3 , R 4 For methyl) 0.73g (2.66mmol), obtain 0.38g white solid 2, yield: 32.2%; m.p.162~164 ℃. 1 HNMR (CDCl 3 ) δ: δ: 10.54 (s, 1H, 6-fluorouracil), 7.80 (d, 1H, 3-fluorouracil), 7.38 (s, 1H, -CONH), 4.32 (s, 2H, -NCH 2 CO), 3.57(m, 6H, -Si(OCH 2 -) 3 ), 3.02(q, 2H, -CONHCH 2 CH 2 ), 2.75(m, 6H, -N(CH 2 ) 3 ), 1.49 (m, 2H, -CH 2 CH 2 CH 2 ), 1.23(m, 9H, -Si(OCHCH 3 ) 3 ), 0.24 (m, 2H, -CH 2 Si). ESI-MS: 445.7 [M+H] + .AnalCalcd for C 18 h 29 N 4 o 6 FSi(444.51): C 48.64, H 6.57, N 12.61; Found: C 49.01, H 6.83, N 12.82
specific Embodiment 3
[0038] γ-[N-[1-oxo-2-[1-(5-fluoro-uracilyl)]propyl]amino]azasilatricyclo(3)
[0039] According to the preparation method of compound (1), drop into 0.5g (2.47mmol) compound A (wherein R 1 is methyl) and DCC 1.0g and p-nitrophenol were added to dry 15ml DMF, heated to 40°C, reacted for 1h, added compound B (where R 2 , R 3 , R 4 for hydrogen) 0.58g (2.66mmol), to obtain 0.19g white solid 3, yield: 18.4%, m.p.158~160℃. 1 H-NMR (CDCl 3 )δ: 10.44 (s, 1H, 6-fluorouracil), 7.68 (d, 1H, 3-fluorouracil), 7.18 (s, 1H, -CONH), 4.09 (m, 1H, -NCHCO), 3.60 (t, 6H ,-Si(OCH 2 -) 3 ), 3.10 (q, 2H, -CONHCH 2 CH 2 ), 2.78(t, 6H, -N(CH 2 ) 3 ), 1.69 (m, 3H, -NCHCH 3 CO), 1.50 (m, 2H, -CH 2 CH 2 CH 2 ), 0.22 (m, 2H, -CH 2 Si).ESI-MS(70V): 417.2[M+H] + .Anal.Calcd for C 16 h 25 N 4 o 6 FSi*0.5H 2 O(425.46): C 45.17, H 6.15, N 13.17; Found: C 45.39, H 6.26, N 13.53
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com