Detecting method for RNA isothermal transcription amplification and its reagent kit
A detection method and RNA polymerase technology are applied in the improvement field of RNA amplification detection technology, and can solve the problems of complicated operation, inability to achieve quantitative detection level, expensive detection instruments and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
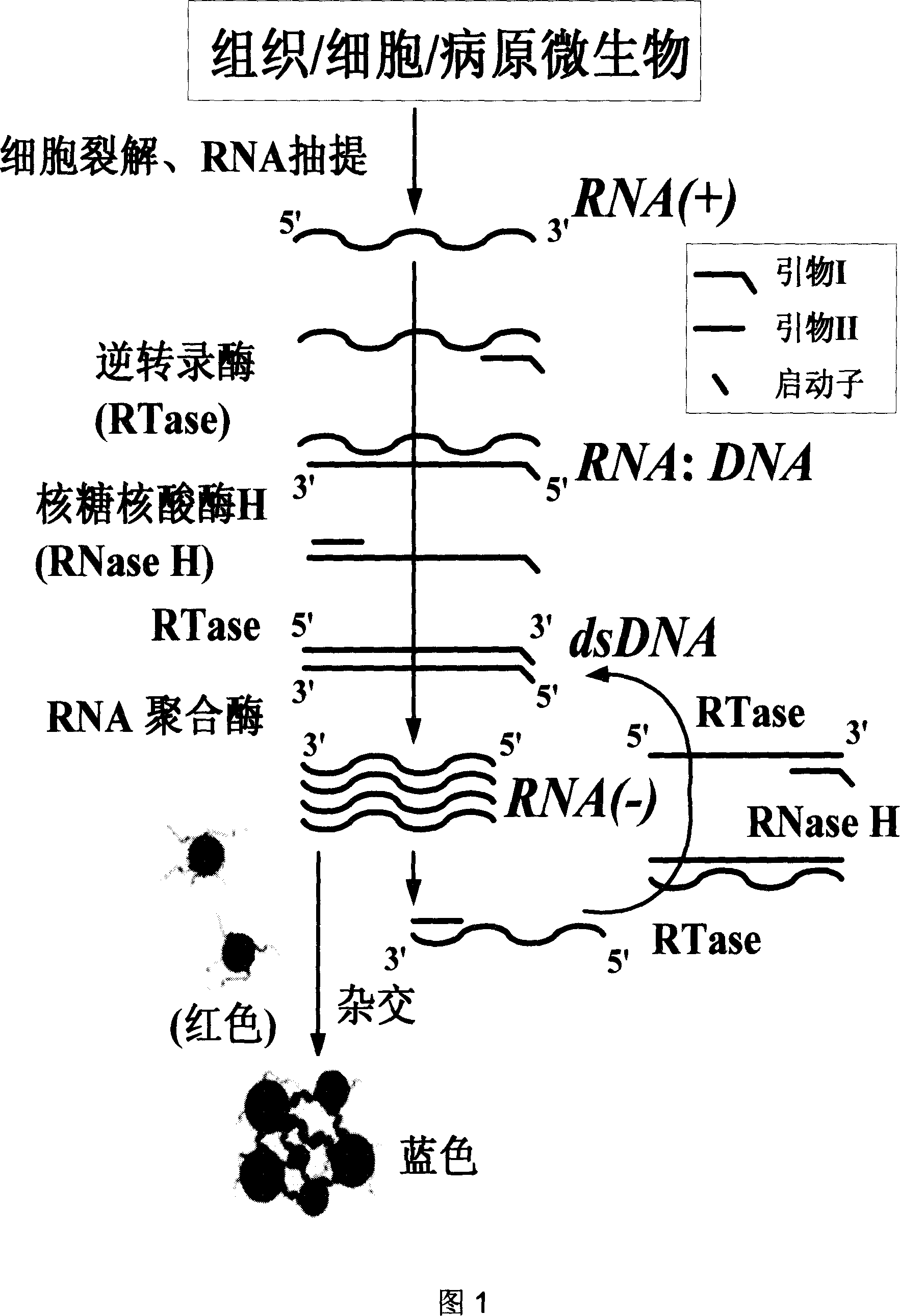
[0073] NITAG detection of RNA samples
[0074] The implementation of the present invention is illustrated below by introducing the detection of hepatitis C virus (HCV) RNA samples.
[0075] Take 100 μL of HCV clinical serum-positive samples, and extract HCV RNA samples according to the instructions of Trizol (Invitrogen, USA) reagent.
[0076] Design of primers and probes: According to literature reports and HCV nucleotide sequence alignment, design isothermal amplification primers and alkylthiolated oligonucleotide probes in the relatively highly conserved 5′-UTR region, upstream primer: 5′ -GAG TGT CGT GCA GCC TCC A-3', downstream primer: 5'- AAT TCT AAT ACG ACT CAC TAT AGG G CAC TCG CAA GCA CCC TAT CA-3', where the underline is the promoter sequence that can be recognized by T7 RNA polymerase; 5'-end thiolated oligonucleotide probe: 5'-HS-(CH 2 ) 6 -[TAC ACC GGA ATT GCC AGG ACG AC]-3’; 3’-Hexylthiolated oligonucleotide probe: 5’-[TGG GTC GCG AAA GGC CTT GTG G]-(CH 2 ...
Embodiment 2
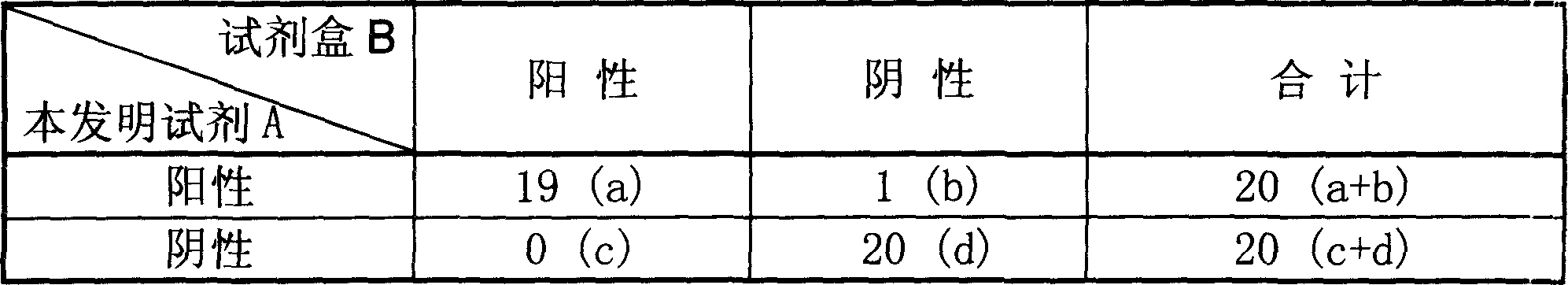
[0081] Adopt the HCV detection kit (A) that assembles according to the present invention, detect 20 HCV positive serum samples and 20 negative serum samples in clinical, simultaneously with the HCV detection kit (B) of Gen-Probe company based on TMA technology For detection comparison, the operation method of the kit of the present invention is according to Example 1, the kit of Gen-Probe Company is stored at -70°C, the operation is carried out according to the instructions and detected by a chemiluminescent detection instrument, and the results are shown in Table 1.
[0082] The overall coincidence rate of the results of the method of the present invention and the TMA technique reached 97.5% in 40 samples, and the results were basically consistent. Wherein 20 routine HCV negative serum samples both detection results are identical; For 20 routine HCV positive serum samples, 19 routine both results meet, 1 routine is positive reagent of the present invention, TMA technology reag...
Embodiment 3
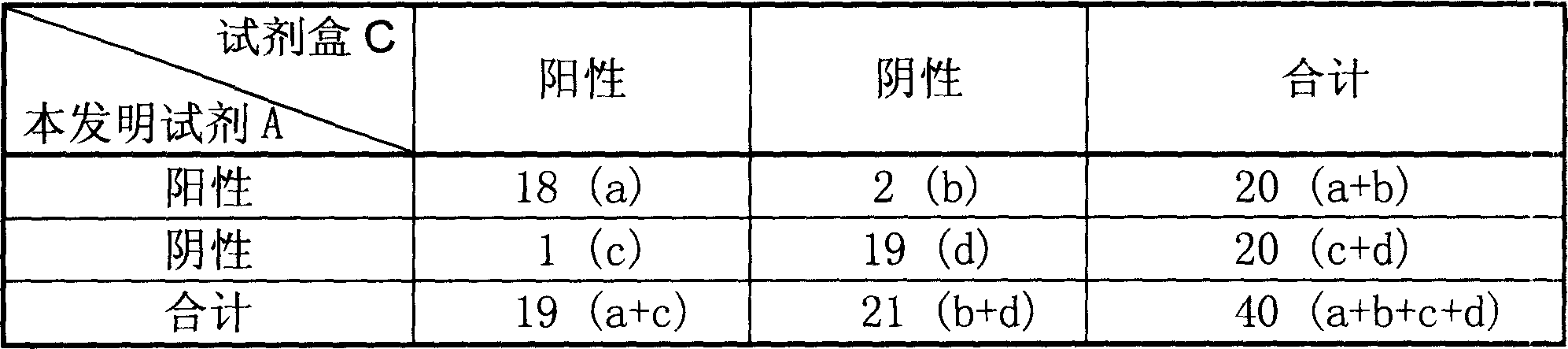
[0090] Adopt the HCV detection kit (A) that assembles according to the present invention, detect 20 parts of HCV positive serum samples and 20 parts of negative serum samples in the clinic, obtain the fluorescent RT-PCR HCV detection kit (C) of domestic drug certificate simultaneously ) for comparison, the operating method of the kit of the present invention is according to Example 1, the control kit (C) is operated according to the instructions, and finally detected on a real-time fluorescent PCR instrument, the results are shown in Table 2, based on the principle of amplification and detection are different According to the technology of the present invention and real-time fluorescent RT-PCR technology, the overall coincidence rate of detecting 40 cases of HCV serum samples is 92.5%, and the results are basically consistent.
[0091] The HCV detection comparative result of table 2 kit of the present invention and fluorescence RT-PCR control technology
[0092]
[0093] Po...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com