Optical active substitution oxyphosphonate salt acetate and its use
A technology of phosphine oxide group and oxo propoxy group is applied in the field of high-efficiency optical resolution of substituted phosphinyl acetic acid, which can solve the problems of high solvent consumption and low yield of the optical resolution method, and achieves reduction of environmental pollution, Improve split yield, simple and efficient recovery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
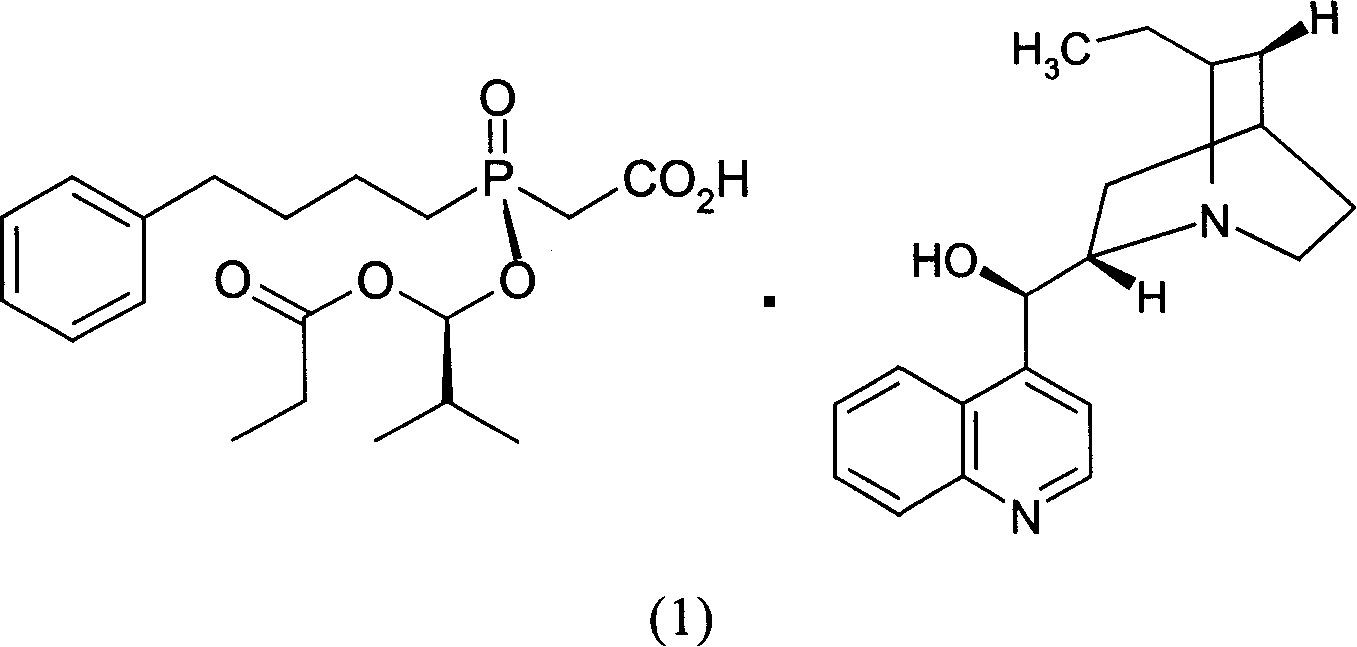
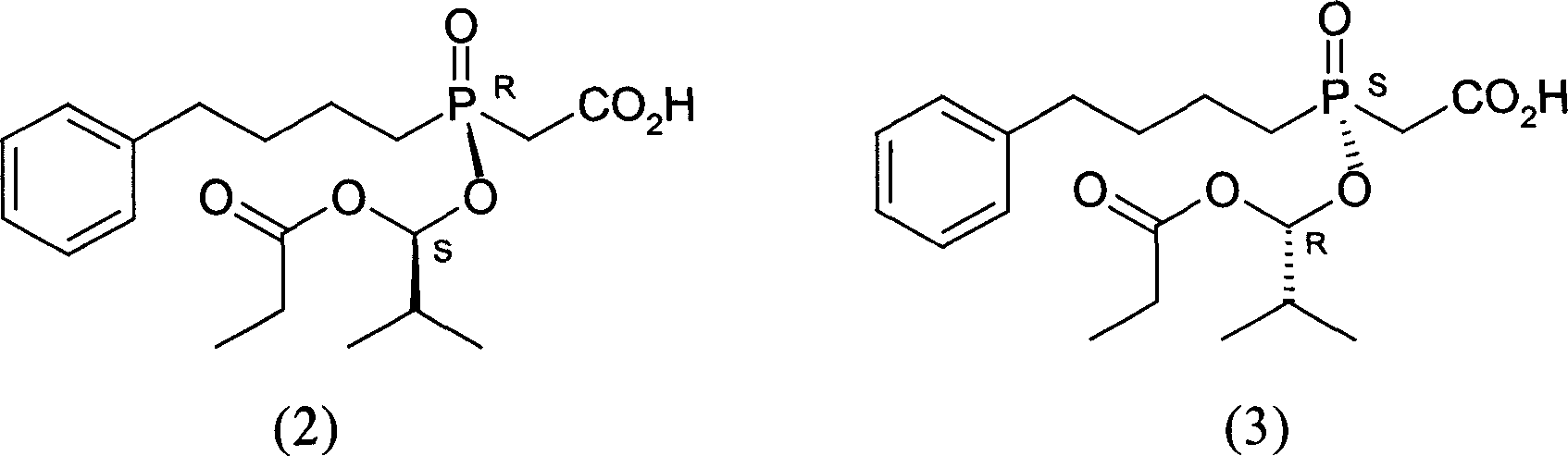
[0037] 10,11-Dihydrocinchonidine (4) (3.56g, 12mmol) and ethyl acetate (30ml) were mixed and stirred to form a suspension, and then added with [(R)-[(1S)-2-methyl Base-1-(1-oxopropoxy)propoxy](4-phenylbutyl)phosphinyl]acetic acid (2) and [(S)-[(1R)-2-methyl-1- (1-oxopropoxy)propoxy](4-phenylbutyl)phosphinyl]racemate (7.68g, 20mmol) composed of acetic acid (3), heated to reflux under stirring Dissolved and filtered while hot. Add seed crystals to the filtrate, cool and crystallize. Suction filtration and vacuum drying gave the salt (1) generated from (2) and 10,11-dihydrocinchonidine (4) as a white solid (4.3g): melting point 125-126°C, [α] 20 D -31.7° (c=1, MeOH). A part of it was acidified and freed with dilute hydrochloric acid, and then extracted with dichloromethane to obtain oil (2): [α] 20 D +45.2° (c=1, EtOAc), the ee value determined by chiral stationary phase HPLC is 97.3%.
[0038] Get above-mentioned salt (1) generated by (2) and 10,11-dihydrocinchonidine (4)...
Embodiment 2
[0042] 10,11-Dihydrocinchonidine (4) (7.11g, 24mmol) and ethyl acetate (68ml) were mixed and stirred to form a suspension, and then added by [(R)-[(1S)-2- Base-1-(1-oxopropoxy)propoxy](4-phenylbutyl)phosphinyl]acetic acid (2) and [(S)-[(1R)-2-methyl-1- (1-Oxopropoxy)propoxy](4-phenylbutyl)phosphinyl]acetic acid (3) racemate (11.53g, 30mmol), stirred and heated until all solids were dissolved. Cool and crystallize. After suction filtration and vacuum drying, the salt (1) formed from (2) and 10,11-dihydrocinchonidine (4) was obtained as a white solid (5.88 g): melting point 122.1-125.4°C.
[0043] Take the above-mentioned salt (1) generated by (2) and 10,11-dihydrocinchonidine (4), and recrystallize once in ethyl acetate to obtain purified salt (2) and 10,11-dihydrocinchonidine The salt (1) formed by hydrocinchonidine (4) is a white solid with a melting point of 123.4-126.5°C.
Embodiment 3
[0045] 10,11-Dihydrocinchonidine (4) (2.96g, 10mmol) and ethyl acetate (60ml) were mixed and stirred to form a suspension, and then added by [(R)-[(1S)-2- Base-1-(1-oxopropoxy)propoxy](4-phenylbutyl)phosphinyl]acetic acid (2) and [(S)-[(1R)-2-methyl-1- (1-Oxopropoxy)propoxy](4-phenylbutyl)phosphinyl]acetic acid (3) racemate (7.68g, 20mmol), stirred and heated until all solids were dissolved. Cool and crystallize. Suction filtration and vacuum drying gave the salt (1) generated from (2) and 10,11-dihydrocinchonidine (4) as a white solid (3.13g): melting point 124.9-126°C, [α] 20 D -29.6° (c=1, MeOH).
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com