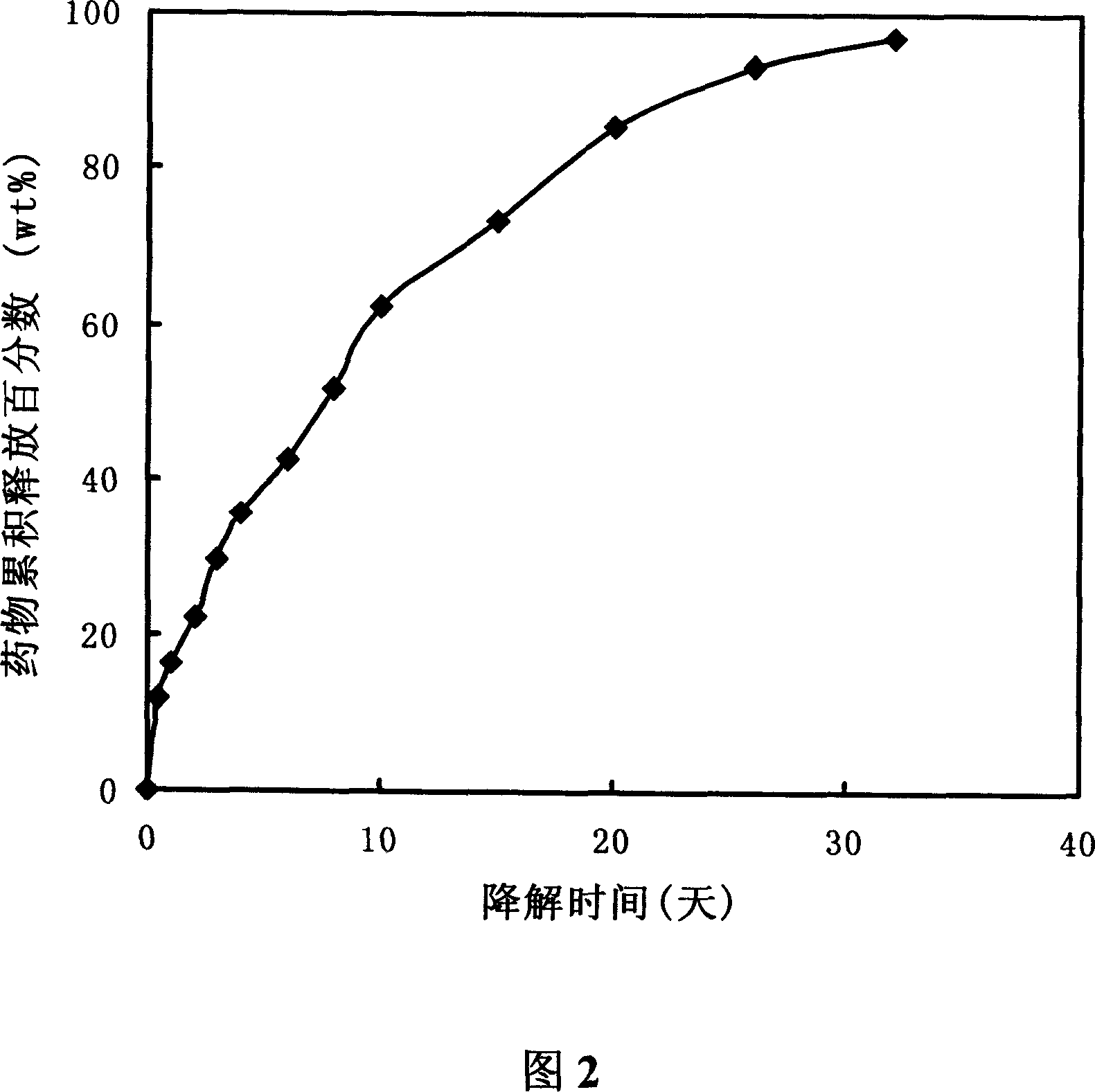
Injectable block copolymer hydrogel of temperature sensibility(epsi - caprolactone - glycolide)- polyethyleneglycol
A copolymer and temperature-sensitive technology, applied in the direction of organic active ingredients, medical preparations of non-active ingredients, peptide/protein ingredients, etc., can solve problems such as limited application, adverse tissue reactions and side effects, and slow degradation rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Synthesis of P(ε-CL-GA)-PEG-P(ε-CL-GA) Triblock Copolymer
[0022] In a 100 ml reaction vessel equipped with a magnetic stirrer, add 20 grams of polyethylene glycol with a molecular weight of 1540, 40 grams of ε-caprolactone, and 4 grams of glycolide, and then inject 0.5 ml of caprylic acid with a micro syringe Stannous solution (0.1g / ml concentration). The reaction system was decompressed and evacuated at room temperature, and the system was replaced with high-purity nitrogen every half an hour, and this was repeated several times. Polymerization was carried out in an oil bath at 120°C with stirring for 12 hours. After the reaction, the obtained polymer was dissolved in dichloromethane, and then precipitated with a large amount of frozen ether, and the purified polymer was dried in a vacuum oven at 70° C. for 24 hours.
Embodiment 2
[0024] Thermal Transition Properties of P(ε-CL-GA)-PEG-P(ε-CL-GA) Triblock Copolymer
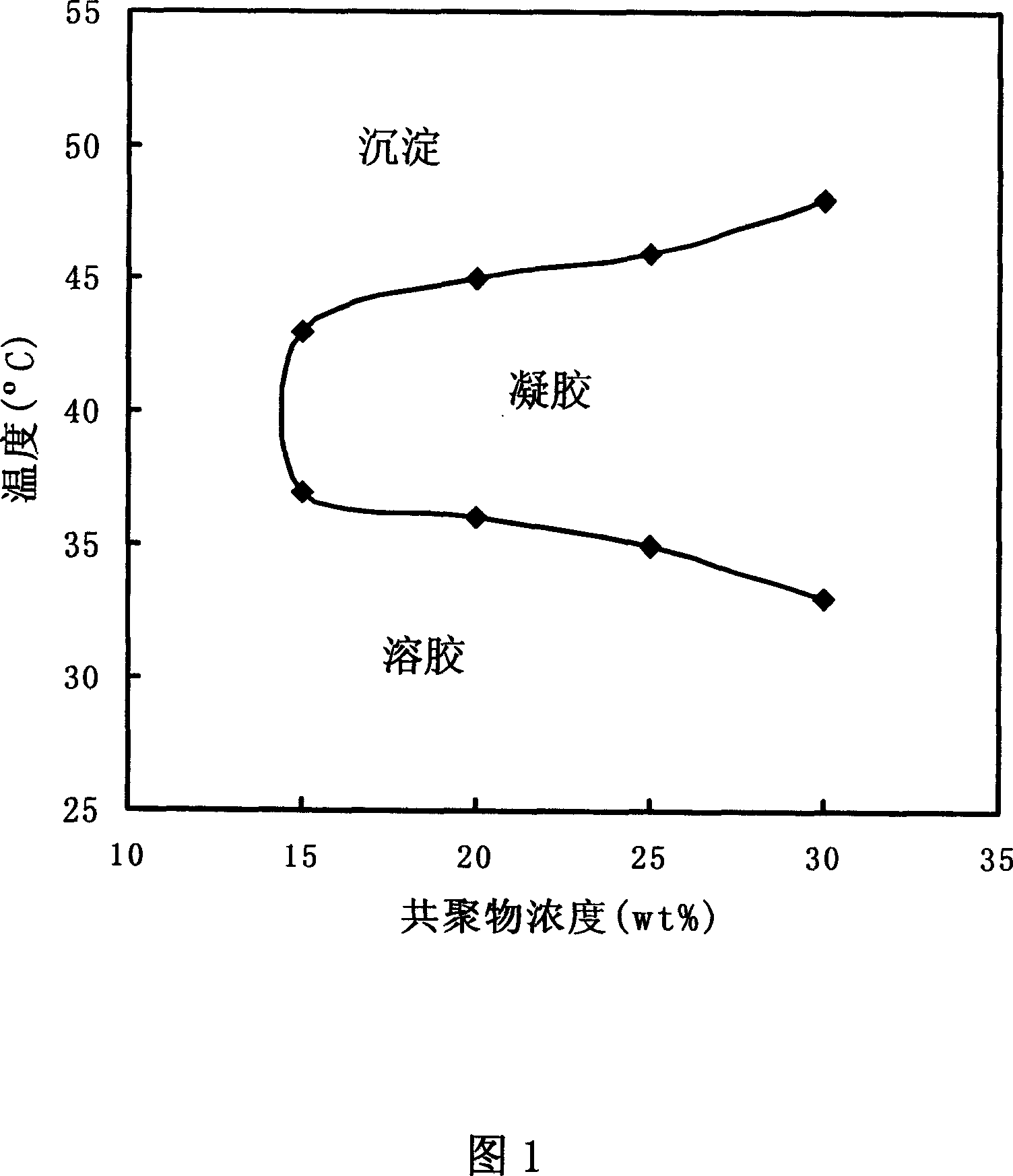
[0025] The sol-gel thermal transition behavior of the triblock copolymer aqueous solution in Example 1 was measured at different concentrations. Aqueous solutions of triblock copolymers with different concentrations were prepared at low temperature, and their viscosity changes between 20-50°C were observed. Gel is defined as the state in which the solution does not flow when the aqueous solution containing the triblock copolymer is inverted. FIG. 1 is a phase diagram showing the sol-gel transition of the triblock copolymer aqueous solution in Example 1. FIG. It can be seen from the phase diagram that when the concentration is above 25wt%, the aqueous solution of the triblock copolymer is a flowable liquid at or below room temperature, but exists in a stable gel state at human body temperature.
Embodiment 3
[0027] Thermosensitivity of ABA Type P(ε-CL-GA)-PEG-P(ε-CL-GA) Triblock Copolymers with Different Compositions
[0028] Using the synthesis method in Example 1, a series of tri-block copolymers with different compositions were synthesized using polyethylene glycol with a molecular weight of 1540 as the hydrophilic segment. The raw material composition ratio of P(ε-CL-GA)-PEG-P(ε-CL-GA) triblock copolymer with reversible thermal transition behavior is shown in the following table:
[0029] Sample No.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com