Method for making high purity cardiomyocyte preparations suitable for regenerative medicine
A technology of cardiomyocytes and cells, applied in the field of preparing high-purity cardiomyocytes suitable for regenerative medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 5
[0080] Examples of direct differentiation techniques are provided in Example 5. First, pPS cells are harvested from expanded pPS cell cultures (preferably feeder-free) and seeded onto a substrate or matrix that is adherent to undifferentiated hES cells and compatible with cardiomyocyte differentiation. Examples are: 0.5% gelatin, 20 μg / mL fibronectin, or Matrigel . The substrate or matrix can be coated on the surface of the culture vessel; or in some cases, the matrix can be part of a particle or network carrier that is present throughout the culture environment. When using gelatin, cell adhesion can be promoted by preincubating the matrix with serum and then washing away the serum before seeding the cells. If desired, pPS cells can be plated on a substrate - for example in continuous culture for an appropriate period of time (ie, 4-8 days) in a medium similar to that used to expand undifferentiated forms of pPS cells - before initiating differentiation. This usually allo...
Embodiment 1
[0143] Example 1: Differentiation from hES cells into cardiomyocytes
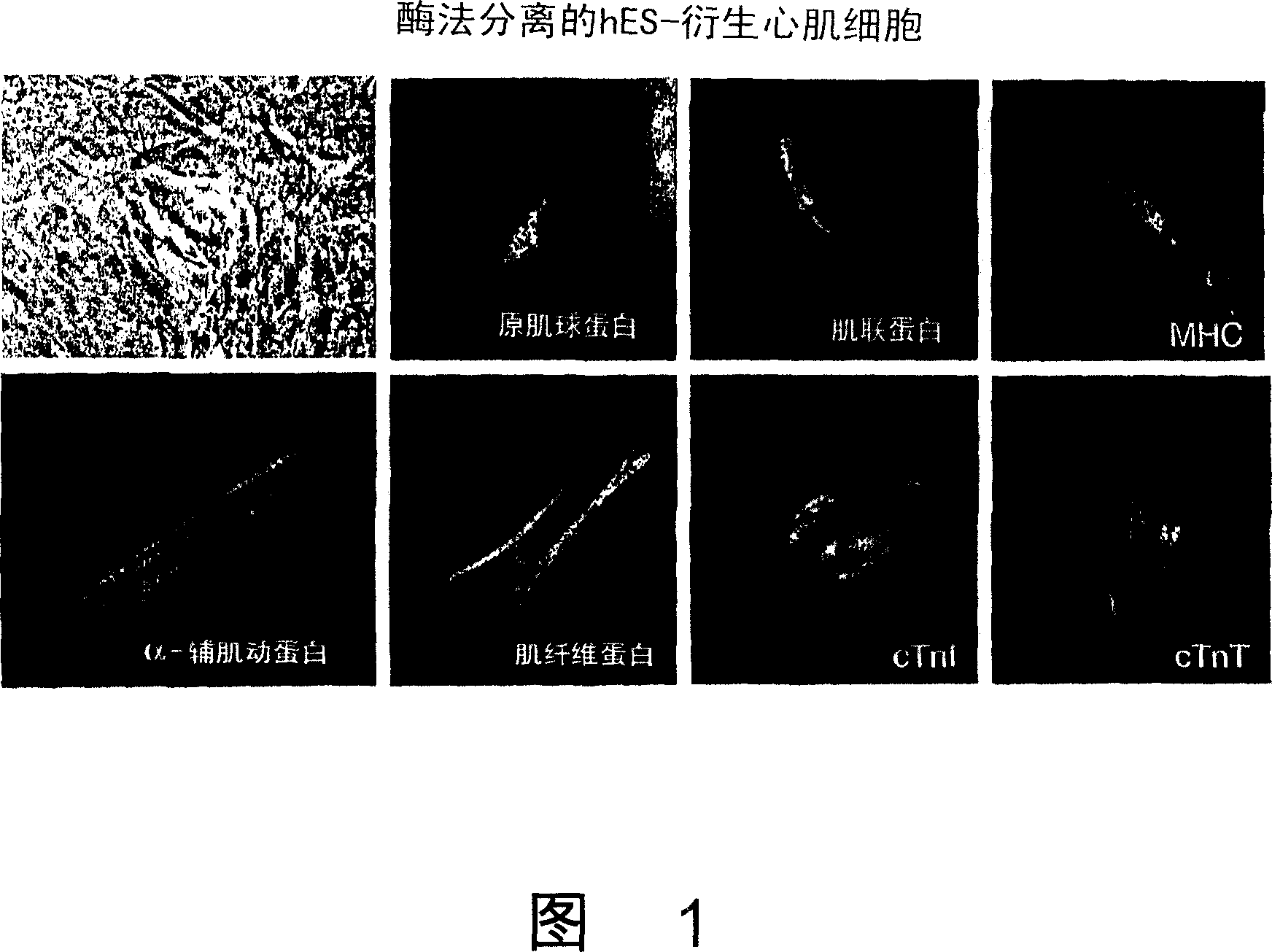
[0144] The hES cell lines H1, H7, H9 and H9.2 (H9-derived clonal lines) were first established on feeder cells and then maintained under feeder-free conditions (as described in WO 01 / 51616). hES cells were cultured in suspension to initiate differentiation to form embryonic bodies. After 4 days of culture in suspension, EBs were transferred to gelatin-coated plates or chambered slides. Beating cardiomyocytes were mechanically dissociated from EB outgrowths on days 15-29 of differentiation, collected and washed. All hES cell lines tested were able to generate beating cardiomyocytes, even after maintenance for more than 50 passages (~260 population doublings), although some cell lines (eg, H7) produced more than others.
[0145] Figure 1 shows the expression of sarcomeric myosin heavy chain (MHC), titin, tropomyosin, α-sarcomeric actinin, myofibers after suspending and reseeding cells with collagenase B Pr...
Embodiment 2
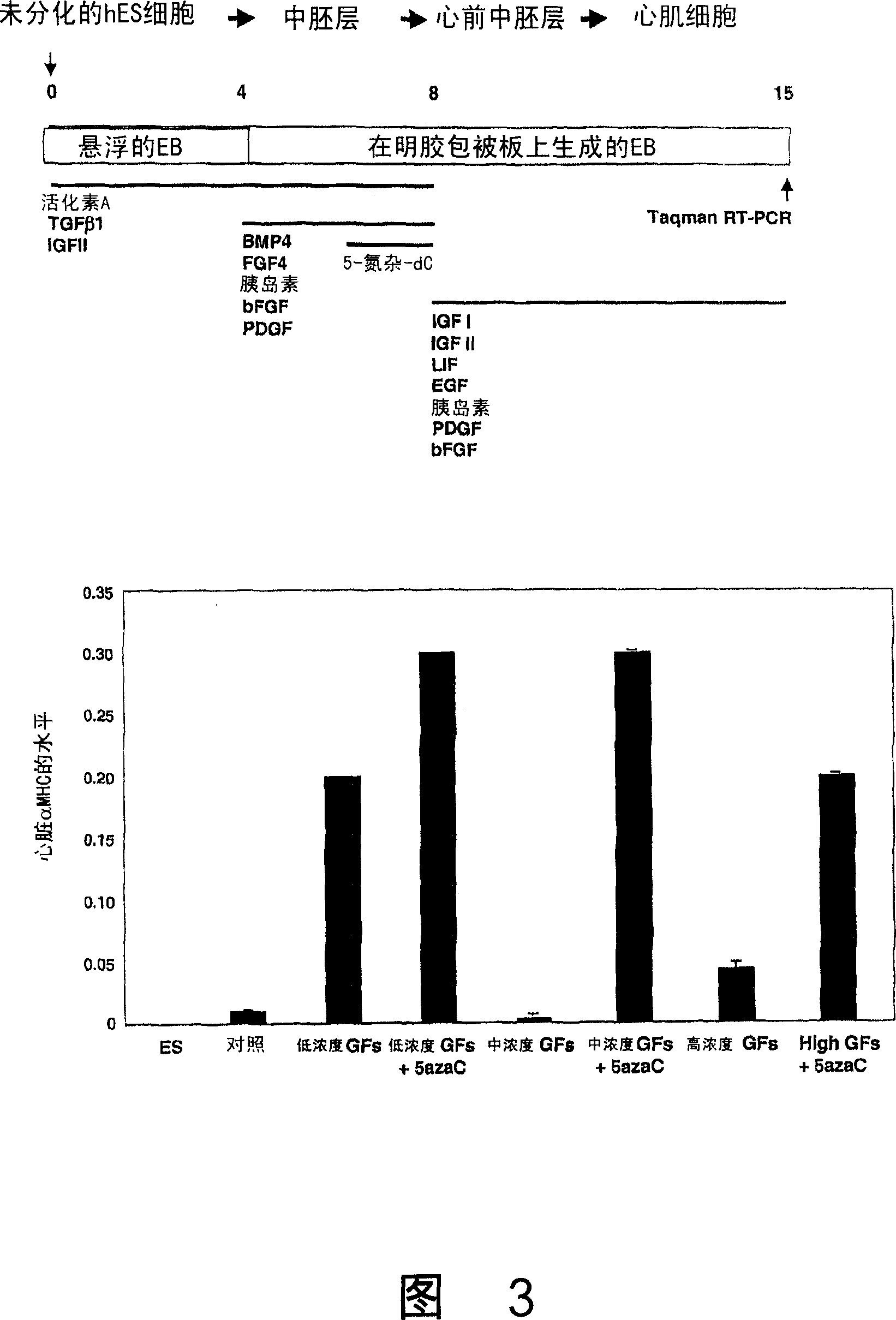
[0149] Example 2: Factors that promote cardiomyocyte differentiation
[0150] Embryos produced by the H1 or H9 lines were treated with 5-aza-deoxy-cytidine, a cytidine analog that affects DNA methylation, on days 1-4, 4-6, or 6-8 of differentiation small body. Cells were harvested on day 15 and analyzed for cardiac MHC by real-time RT-PCR. On days 6-8, 1-10 μM of 5-aza-deoxy-cytidine significantly increased cardiac α-MHC expression, which correlated with an increased proportion of beating areas in culture.
[0151] Other agents were tested for their ability to induce cardiomyocyte differentiation, including: dimethyl sulfoxide (DMSO) and all-trans retinoic acid (RA). Embryoid bodies treated with 0.5% DMSO on days 0-4 produced fewer beating areas than untreated cultures. There were no beating cells in cultures treated with 0.8% or 1% DMSO, while 1.5% DMSO was in fact toxic to the cells. Treatment with DMSO also resulted in a significant decrease in α-MHC expression compared...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com