Peptides and related molecules that bind to tall-1
A composition, f12 technology, applied in the direction of hybrid peptides, fusion polypeptides, peptide/protein components, etc., can solve the problems of recombinant or modified proteins that have not yet disclosed peptide regulators
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
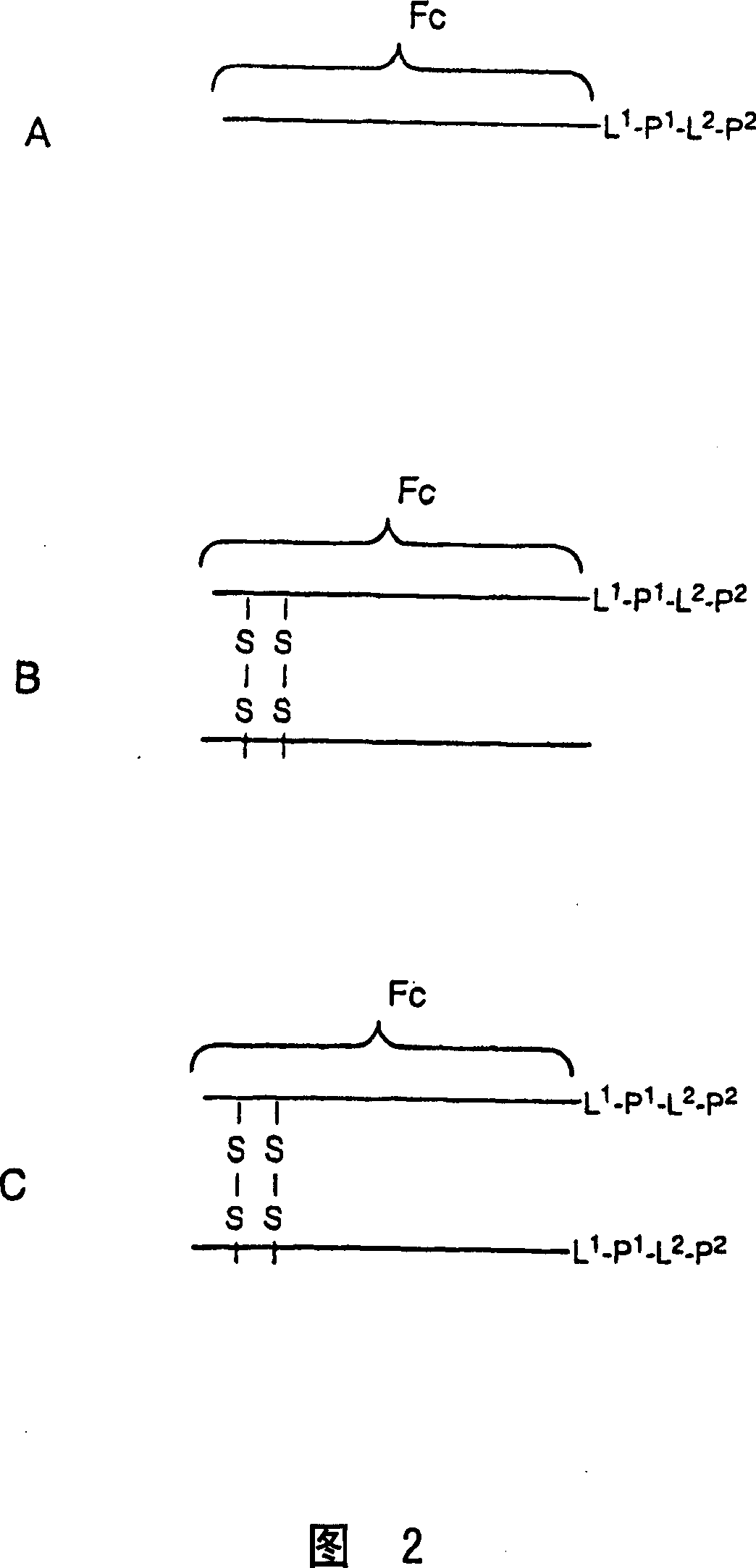
[0423] The inventors have determined the preferred structures of the preferred peptides listed in Table 4 below. The symbol "A" may be any linker disclosed herein, or may simply represent an ordinary peptide bond (ie no linker present). Tandem repeats and adapters are shown separately for clarity.
[0424] Table 4 - Preferred Embodiments
[0425] (Original page 59)
[0426] "V 1 " is the Fc domain as defined hereinbefore. In addition to those listed in Table 4, the inventors also proposed heterodimers, wherein each chain of the Fc diabody is associated with a different peptide sequence linked; eg, wherein each Fc is linked to a different sequence selected from Table 2.
[0427] All compounds of the present invention can be prepared by the methods disclosed in PCT Application No. WO 99 / 25044.
[0428] The present invention will be further described below by means of practical examples, which are illustrative rather than limiting.
example 1
[0430] peptide
[0431] Peptide Phage Display
[0432] 1. Magnetic Bead Preparation
[0433] A. Fc-TALL-1 immobilized on magnetic beads
[0434] Recombinant Fc-TALL-1 protein was immobilized on Protein A Dynabeads (Dynal) at a concentration of 8 micrograms of Fc-TALL-1 per 100 microliters of magnetic bead stock purchased from the manufacturer. By drawing the beads to the side of the tube using a magnet and removing the liquid, the beads were washed twice with phosphate buffered saline (PBS) and resuspended in PBS. Fc-TALL-1 protein was added to the washed magnetic beads at the above concentrations and incubated with rotation at room temperature for 1 hour. Fc-TALL-1 coated beads were then blocked by addition of bovine serum albumin (BSA) at a final concentration of 1% and incubated overnight at 4°C with rotation. The resulting Fc-TALL-1 coated beads were then washed with PBST (PBS containing 0.05% Tween-20) before performing the screening method.
[0435] B. Negative Scre...
example 2
[0476] peptibody
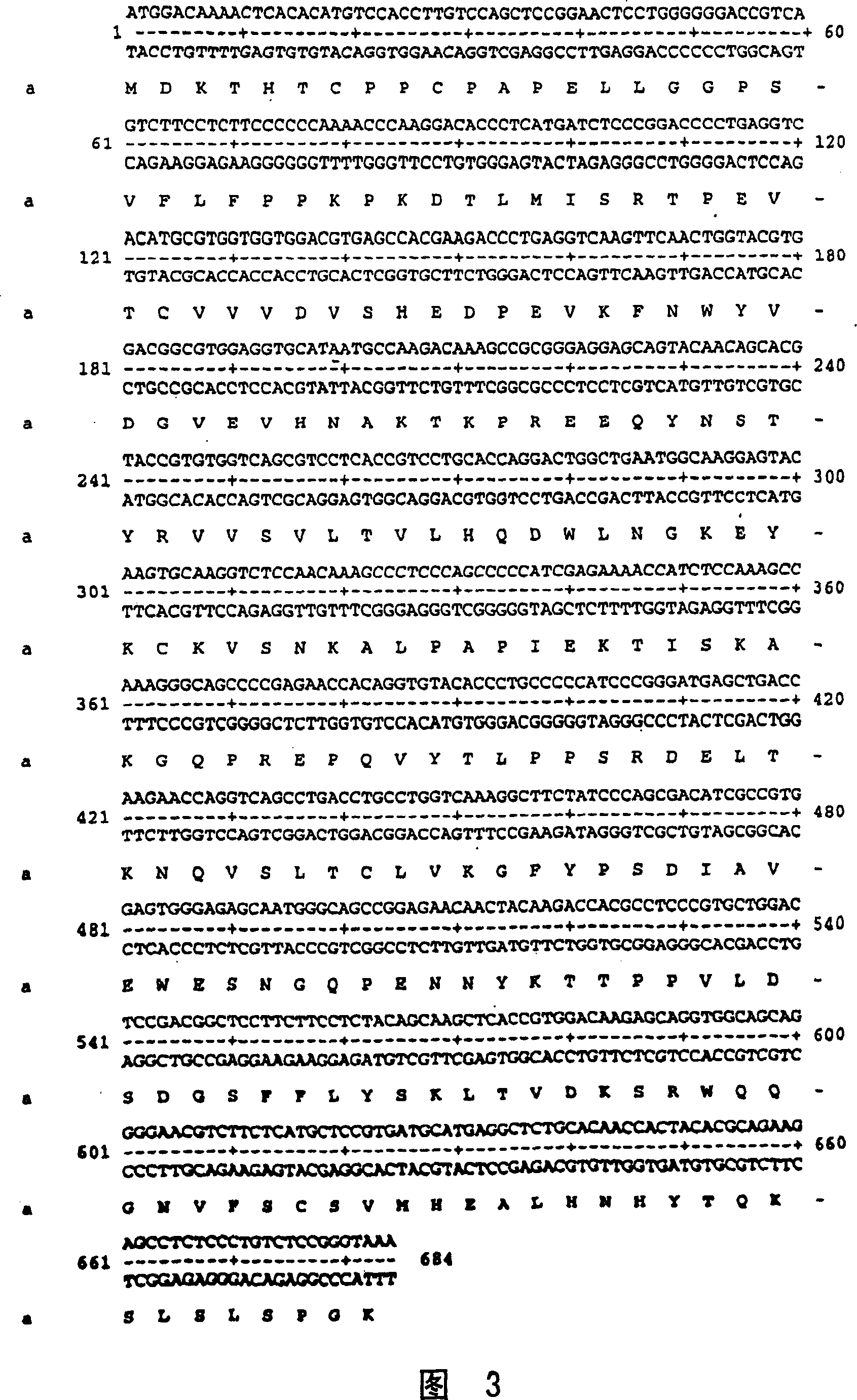
[0477] A set of 12 TALL-1 inhibitory peptibodies (Table 5) was constructed in which one monomer of each peptide was fused in frame to the Fc region of human IgG1. Each of the TALL-1 inhibitory peptibodies was constructed by annealing the oligonucleotide pairs shown in Table 6 to generate a duplex encoding the peptide and a linker comprising 5 glycine residues and a valine residue, appearing as NdeI-SalI fragments. This double-stranded molecule was ligated into a vector containing the human Fc gene (pAMG21 RANK-Fc, as disclosed herein), also digested with NdeI and SalI. The resulting ligation mixture was transformed into E. coli strain 2596 cells (GM221, as disclosed herein) by electroporation. Clones were screened for their ability to produce recombinant protein products and possess gene fusions with the correct nucleotide sequence. One such clone was selected for each peptibody. The nucleotide and amino acid sequences of the fusion protein are shown in ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com