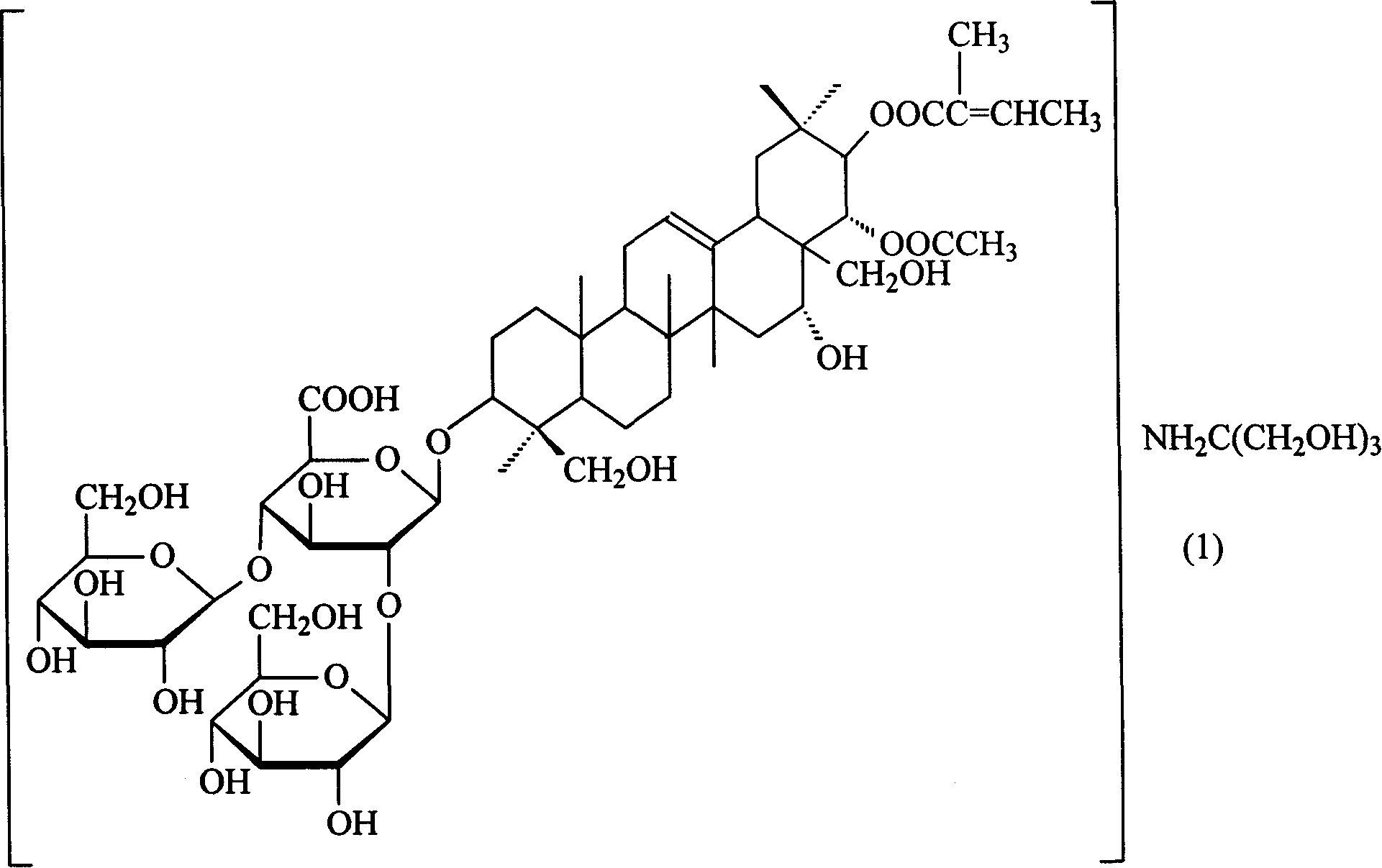
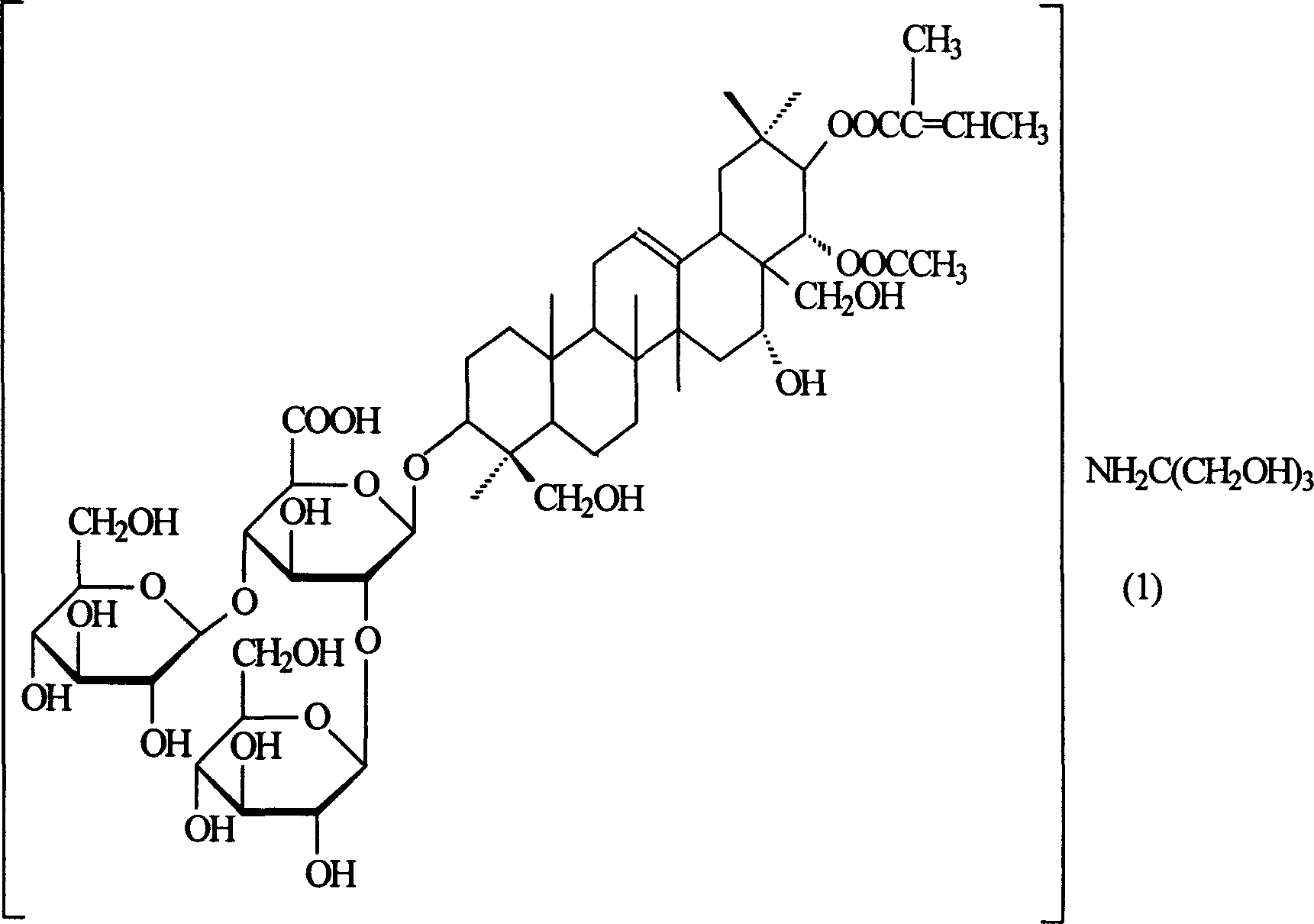
Amino butanetriol aescin and its prepn process
A technique for escin and tromethamine, which is applied to pharmaceutical formulations, medical preparations containing active ingredients, steroids, etc., can solve the hidden dangers of unsafe medicines, poor stability of arginine escin, and formulation molding. Problems such as difficulties in drug storage and transportation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Embodiment 1: Preparation of tromethamine aescin
[0017] Get commercially available escin 113.1g (0.1mol), tromethamine 12.1g (0.1mol) and 1000ml distilled water, stir to react to a clear liquid, evaporate the solvent to dryness under reduced pressure, filter, and wash with absolute ethanol until no It was turbid and dried under reduced pressure at 80°C to obtain 100.2 g of off-white solid with a yield of 80.0%.
[0018] Aescin was used as the reference substance for content determination, and the product content was 99.6%.
Embodiment 2
[0019] Embodiment 2: Preparation of tromethamine aescin
[0020] Get commercially available aescin 11 3.1g (0.1mol), tromethamine 12.1g (0.1mol) and 1000ml distilled water, stir to react to a clear liquid, evaporate the solvent under reduced pressure, add 300ml acetone and no A mixed solvent of water and ethanol (1:1) was ground to obtain an off-white precipitate, which was filtered and dried under reduced pressure at 80° C. to obtain 92.2 g of off-white solid with a yield of 73.6%.
[0021] Aescin was used as the reference substance for content determination, and the product content was 99.3%.
Embodiment 3
[0022] Embodiment 3: Preparation of tromethamine aescin
[0023] Take commercially available escin 113.1g (0.1mol), tromethamine 12.1 (0.1mol) and 1000ml of distilled water, stir the reaction to a clear liquid, and freeze-dry to obtain 122.8g of off-white solid with a yield of 98.1%.
[0024] Aescin sodium was used as the reference substance for content determination, and the product content was 98.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com