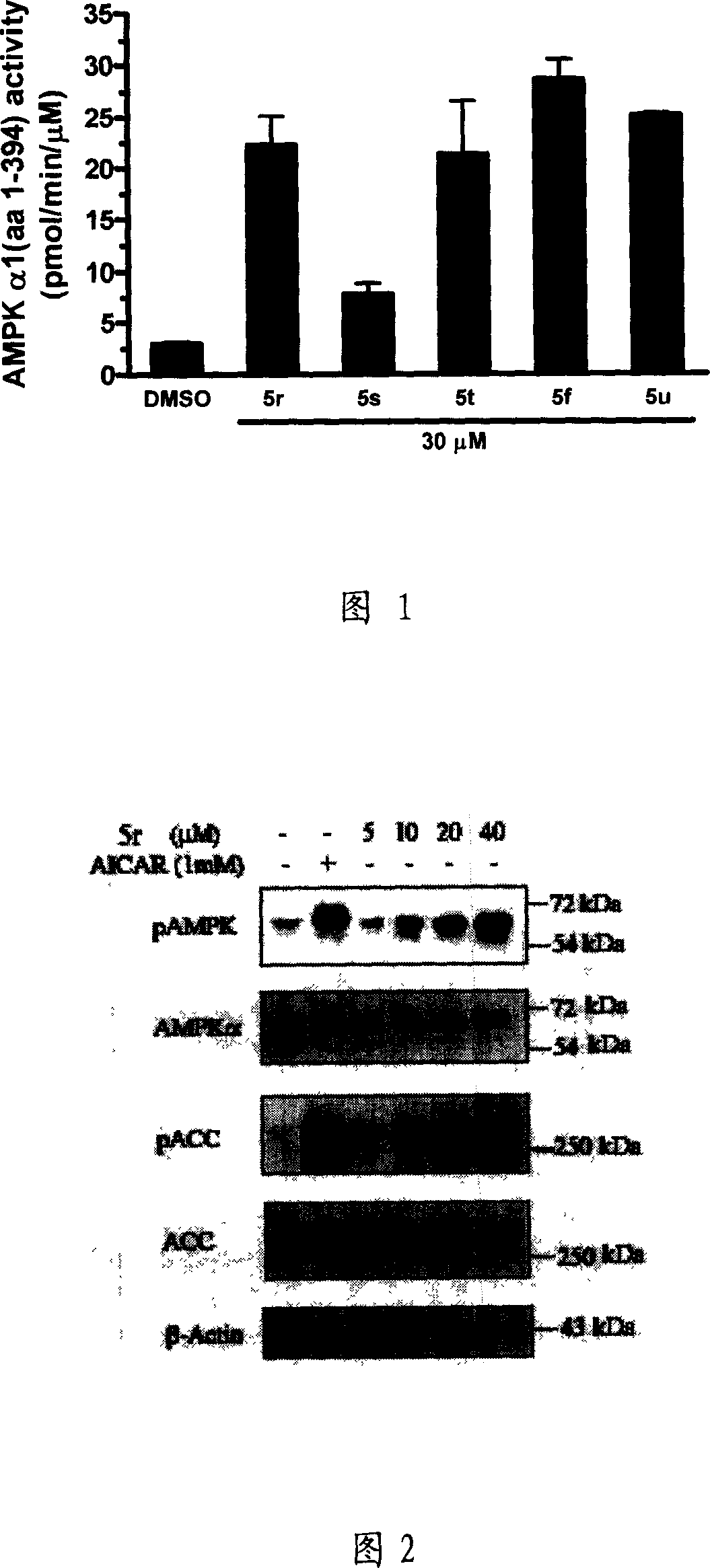
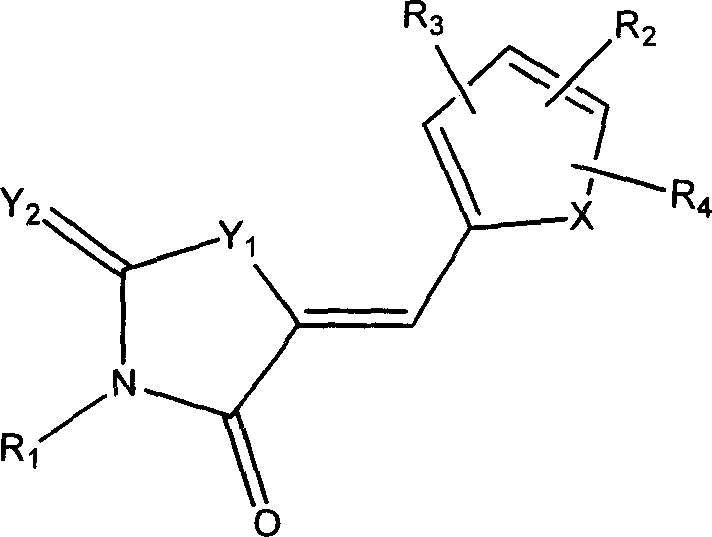
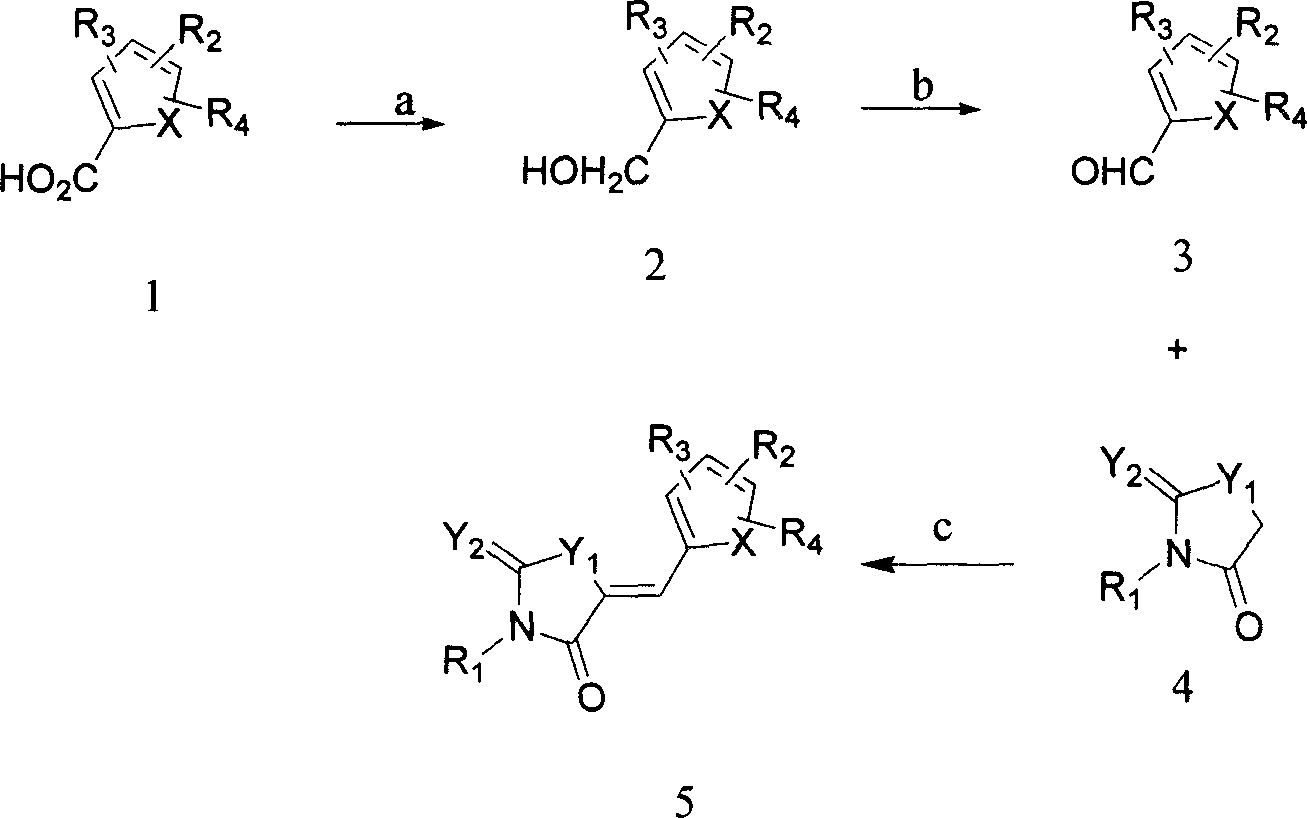
Compound, and its preparing method and use
A compound, alkyl technology, applied in the field of small molecule organic compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1: the preparation of compound 5a
[0044] [Chemical Reaction Formula 4]
[0045]
[0046] According to the above chemical reaction formula:
[0047] Step 1: Add 28mg of AlLiH to a 25ml round bottom flask 4 , disperse it in 10ml of freshly steamed THF, add dropwise 101mg of compound 3a (0.37mmol) in 3ml of freshly steamed THF solution at room temperature, stir the reaction at room temperature (24-26°C) for 30 minutes, and use TLC to track and measure the reaction degree of completion. After the reaction is complete, carefully add 0.5ml of water, continue stirring for 10 minutes, dilute with water, extract with ethyl acetate, and wash the organic phase with anhydrous Na 2 SO 4 After drying and concentration to remove the solvent, 2b was obtained.
[0048] The second step: add 68mg compound 2a, 20ml chloroform in 50ml round bottom flask, add active MnO under stirring 2 122mg, stirred at room temperature (24-26°C) for 5 hours, followed by TLC to deter...
Embodiment 2
[0051] Embodiment 2: the preparation of compound 5b:
[0052] [Chemical Reaction Formula 5]
[0053]
[0054] According to the above chemical reaction formula:
[0055] The first step: Compound 2b was prepared in the same manner as the first step of Example 1, except that the raw material 1a in the first step in Example 1 was replaced by raw material 1b.
[0056] Second step: Compound 3b was prepared in the same manner as the second step of Example 1, except that raw material 2b was used instead of raw material 2a in the second step of Example 1.
[0057] The third step: add 34mg of compound 3b (0.28mmol), 60mg of compound 4b (0.28mmol), 100mg of AcONa, 2ml of glacial acetic acid in a 5ml round bottom flask, reflux under nitrogen protection (100°C to 120°C) for 2 hours, and track the reaction by TLC . After the reaction was completed, it was cooled, filtered, washed and dried in vacuo to obtain 42 mg of compound 5b.
[0058] Compound 5b: 1H NMR (CDCl3, 300MHz) δ1.27~1...
Embodiment 3
[0059] Embodiment 3: the preparation of compound 5c:
[0060] Compound 5c was prepared in the same manner as in Example 1, except that the corresponding carboxylic acid or carboxylic acid ester and carbonyl compound were used as raw materials.
[0061] The structural formula of compound 5c:
[0062]
[0063] Compound 5c: 1 H NMR (DMSO-d 6 , 300MHz) δ2.32(s, 6H), 3.66(s, 3H), 3.75(s, 3H), 3.93(s, 2H), 6.78(d, 1H), 6.93(d, 1H), 7.01(s , 1H), 7.09 (s, 1H), 7.63 (s, 1H), 7.73 (s, 1H), 7.83 (d, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com