Human pancreas glucagon sample peptide-1-derivative, its production and use
A derivative, GLP-1 technology, applied in the direction of glucagon, hormone peptides, specific peptides, etc., can solve the problems of half-life extension, preparation and purification difficulties, etc., and achieve low production cost, low cost, and simplified purification process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
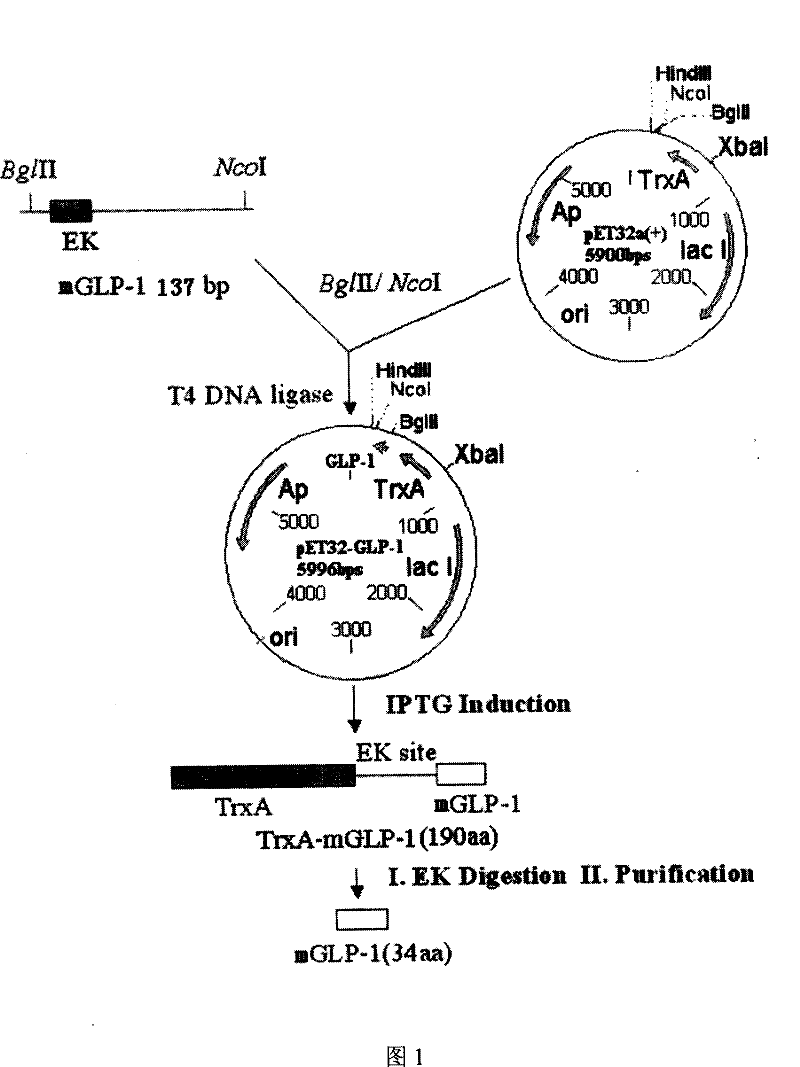
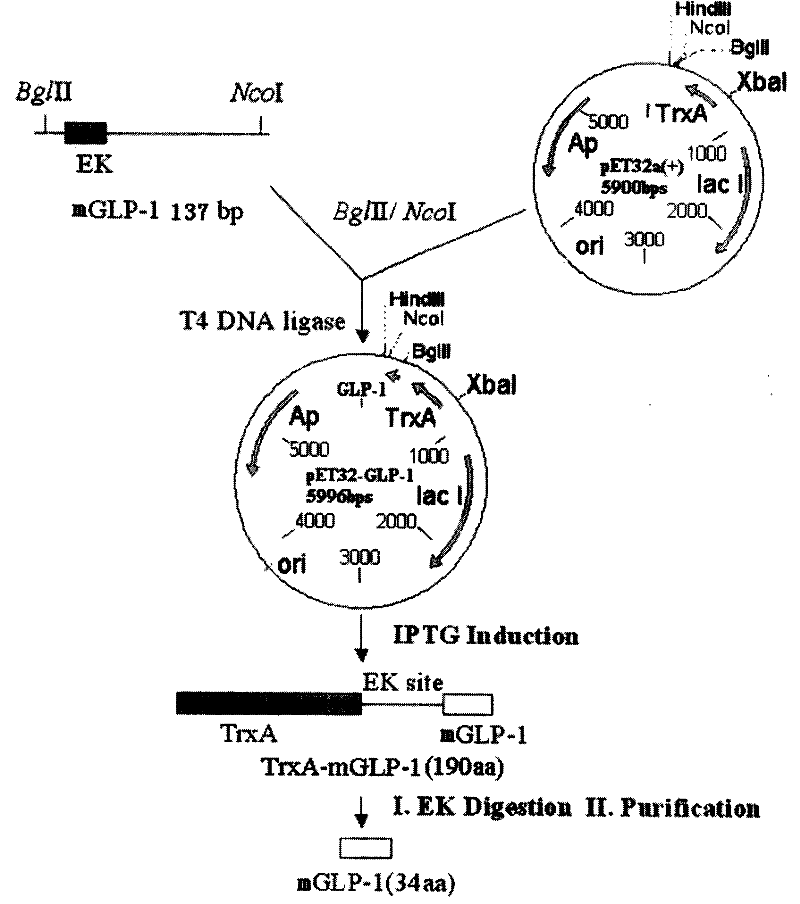
[0028] According to the amino acid sequence of the GLP-1 derivative, the following fragment with a length of 131bp was synthesized, namely Seq ID No.5, using the preferred codons of Escherichia coli:
[0029] ccagatctgg acgacgacga caagcatgcc gaaggcacct ttaccagcga tgtgagcagc 60
[0030] tatctggaag gccaggccgc caaagaattt attgcctggc tggtgaaagg cagaggcnnn 120
[0031] taaccatgga c 131
[0032] Wherein n118=n120=t, n119=g, this fragment contains enterokinase EK site, GLP-1 derivative gene, stop codon TAA and restriction endonuclease BglII and NcoI site;
[0033] The second step places the coding sequence into an expression vector in a manner suitable for expression of the fusion protein:
[0034] The gene fragment obtained in the first step was double-digested with restriction endonucleases BglII and NcoI, purified and recovered, and the Escherichia coli plasmid pET-32a(+) was double-digested with restriction endonucleases BglII and NcoI, and purified Recover the large fragment, mi...
Embodiment 2
[0045] According to the amino acid sequence of the GLP-1 derivative, the following fragment with a length of 134bp was synthesized, namely Seq ID No.6, using the preferred codons of Escherichia coli:
[0046] ccagatctgg acgacgacga caagcatgcc gaaggcacct ttaccagcga tgtgagcagc 60
[0047] tatctggaag gccaggccgc caaagaattt attgcctggc tggtgaaagg cagaggcnnn 120
[0048] nnntaaccat ggac 134
[0049] Wherein n118=n120=t, n119=n121=n122=g, n123=c, this fragment contains enterokinase EK site, human glucagon-like peptide GLP-1 derivative gene, stop codon TAA and restriction endonuclease BglII and NcoI sites;
[0050] Other steps are the same as in Example 1.
[0051] Example 3 Preparation of the GLP-1 derivative of the present invention by DNA recombination technology: the molecular structural formula of the derivative is GLP-1(7-37)-Xaa38-Xaa39 (Seq ID No.3), wherein Xaa38=Gly, Xaa39=Cys , the operation steps are the same as in Example 2, in the Seq ID No.6 gene fragment synthesized ...
Embodiment 3
[0053] The first step is to synthesize a gene fragment according to the amino acid sequence of the GLP-1 derivative:
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com