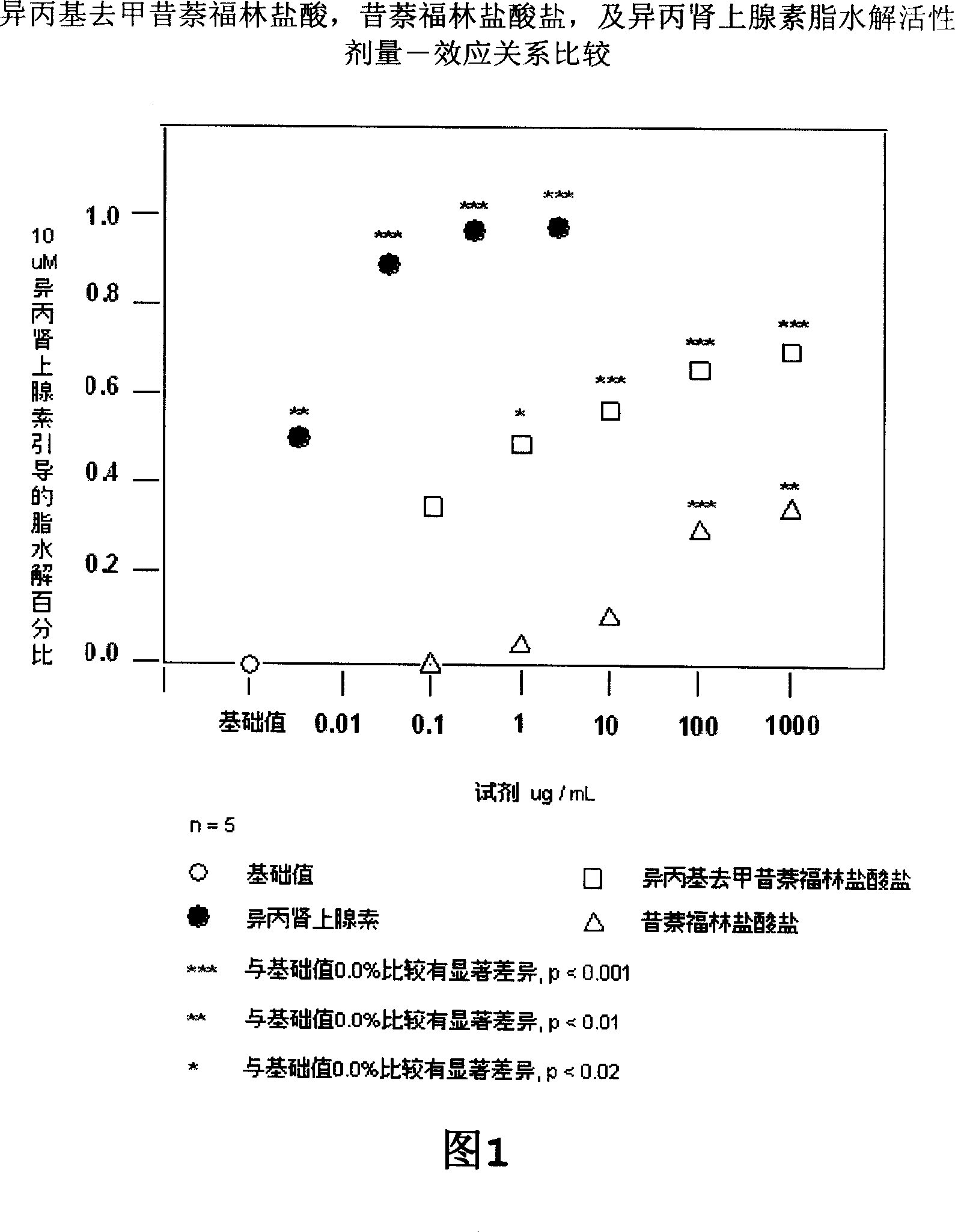
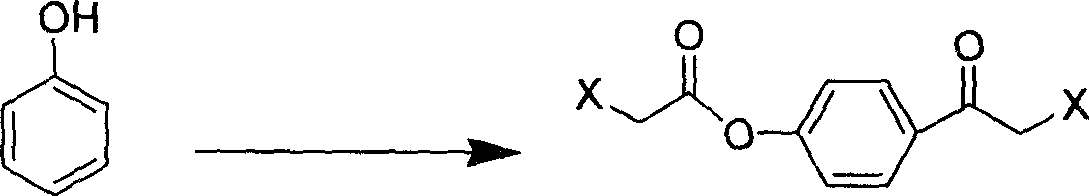Synthesis and uses of synephrine derivatives
A technology of reaction and compound, applied in the field of synthesis and application of synephrine derivatives, can solve problems such as death and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
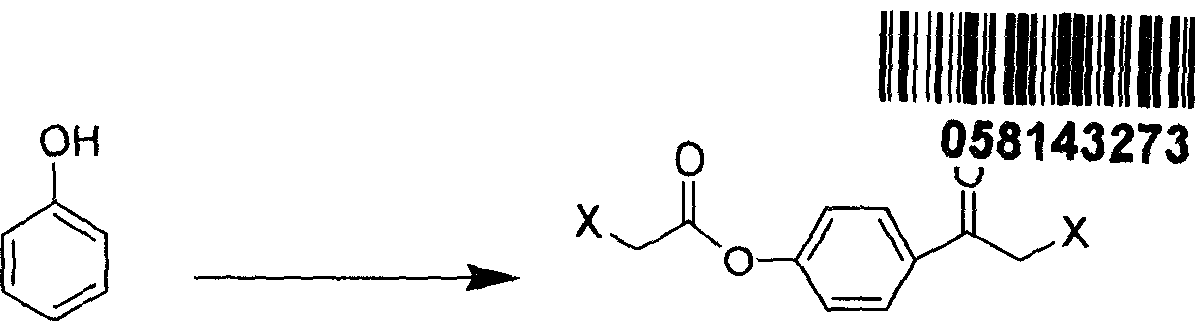
[0155] Novel Synthesis of Novel Compound 2-Chloro-1-(4-Chloroacetoxyphenyl)-Ethanone (An Example of Formula III Where "X" Is Chloro)
[0156] This example provides the synthetic method of new intermediate 2-chloro-1-(4-chloroacetoxybenzene)-ethanone, its structure is as follows:
[0157]
[0158] 9.4 g of phenol was dissolved in 10 ml of dichloroethane, and the resulting solution was cooled with an ice bath. Then add 33.4 g of aluminum trichloride in portions while stirring, and remove the ice bath after adding. Then, a solution of 25.0 g of chloroacetyl chloride dissolved in 15 ml of dichloroethane was added dropwise to the above mixture, and the addition was completed in about 1 hour. There are many gases produced. After the reaction mixture was stirred and reacted at 25° C. for 5 hours, the reaction mixture was poured into dilute glacial hydrochloric acid water while stirring to fully hydrolyze it. The resulting solid suspension was filtered and washed with acid and...
example 2
[0159] Literature preparation of the known 2-chloro-1-(4-hydroxyphenyl)-ethanone (an example of formula VI where "X" is chloro)
[0160] This example provides the literature preparation method of 2-chloro-1-(4-hydroxyphenyl)-ethanone, whose structure is as follows:
[0161]
[0162] 9.4 g of phenol was dissolved in 10 ml of dichloroethane, and the resulting solution was cooled with an ice bath. Then add 33.4 g of aluminum trichloride in portions while stirring, and remove the ice bath after adding. Then, a solution of 17.0 g of chloroacetyl chloride dissolved in 15 ml of dichloroethane was added dropwise to the above mixture, and the addition was completed in about 1 hour. There are many gases produced. The reaction mixture was stirred at 65°C for 12 hours. The reaction mixture was poured into dilute glacial hydrochloric acid water, the insoluble viscous material was extracted with dichloromethane, the dichloromethane solution was dried over anhydrous sodium sulfate, f...
example 3
[0163] Novel preparation of the known 2-chloro-1-(4-hydroxyphenyl)-ethanone by hydrolysis (an example of formula VI where "X" is chloro)
[0164] This example provides a new preparation method of 2-chloro-1-(4-hydroxyphenyl)-ethanone, whose structural formula is as in example 2 above.
[0165] 10 g of 2-chloro-1-(4-chloroacetoxyphenyl)-ethanone prepared in Example 1 was suspended in 50 ml of methanol, and the suspension was cooled by an ice bath. 5% sodium hydroxide solution in equimolar ratio was added dropwise, and after the addition was completed, the mixture was stirred and reacted at room temperature for 0.5 hours. Then most of the solvent was distilled off, and the resulting residue was frozen and crystallized, and 5.9 g of crystals were obtained by filtration, with a yield of 85% and a melting point of 151-152°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com