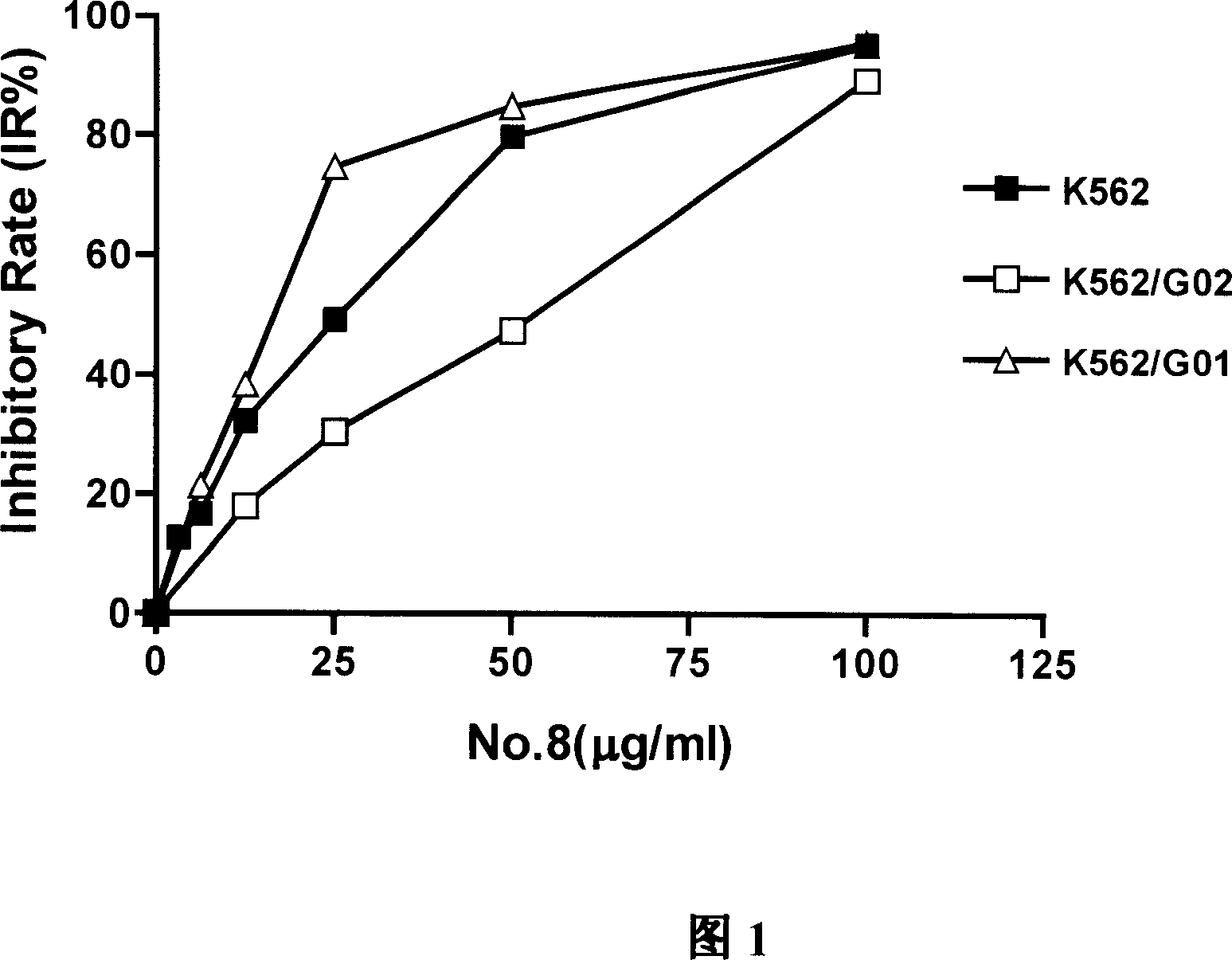
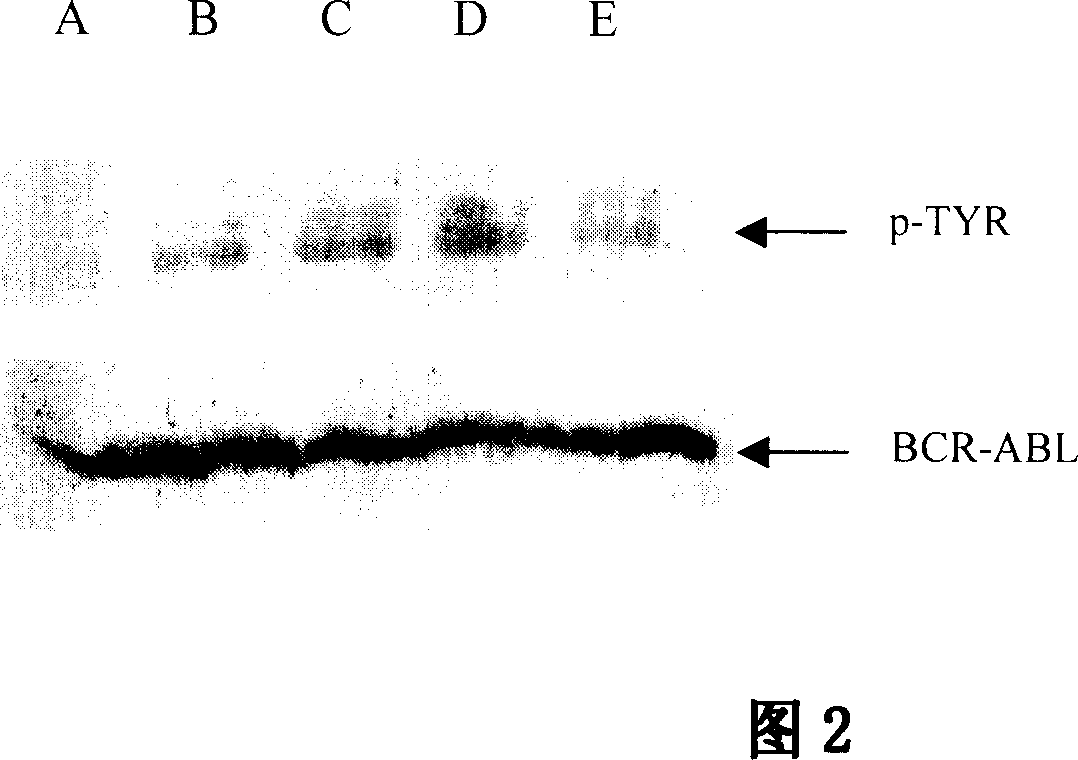
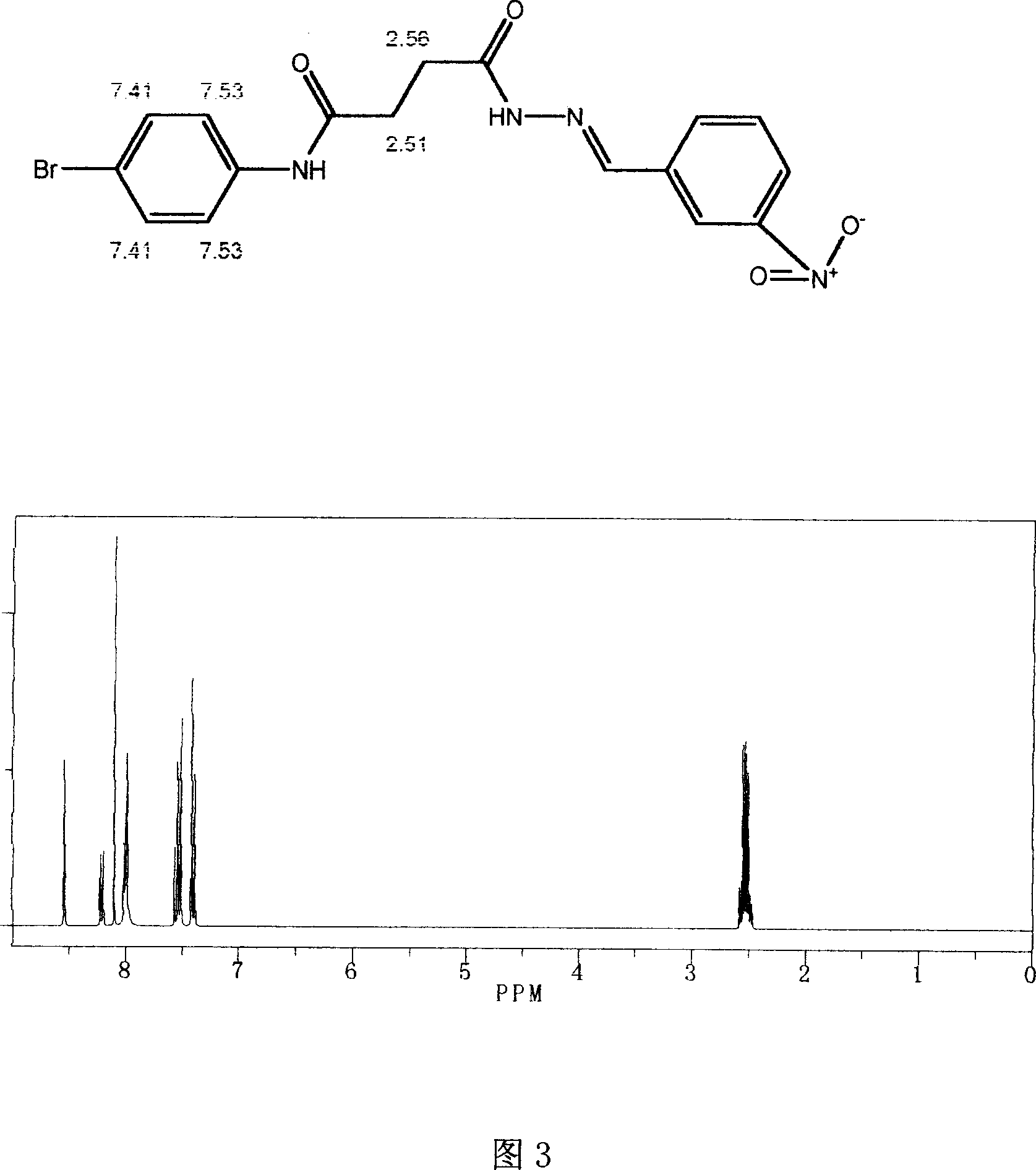
Use of succinyl hydrazine compounds and compositions thereof in preparation of drug for treating leukemia
A compound, leukemia technology, applied in the field of medicine, can solve problems such as drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Preparation of succinic acid mono(p-bromophenyl)amide (compound V)
[0063] p-Bromoaniline (7.08g, 40mmol) and succinic anhydride (4.48g, 40mmol) were dissolved in 300ml chloroform and stirred at room temperature, reacted overnight, and needle-like crystals were precipitated, and the product V9.80g was obtained by filtration, with a yield of 88.44%.
Embodiment 2
[0065] Preparation of 1-(p-bromophenyl)amide 4-methyl succinate (compound VI)
[0066] The product V (5g, 18mmol) and iodomethane (3.33g, 23mmol) were dissolved in 100ml DMF, potassium bicarbonate (2.35g, 23mmol) was added, and stirred at room temperature for 16 hours. The reaction solution was diluted with dichloromethane, washed repeatedly, Washed with sodium bicarbonate and dried over anhydrous sodium sulfate. The solvent was distilled off to obtain 5.0 g of product VI with a yield of 95.45%.
Embodiment 3
[0068] Preparation of succinic acid 1-(p-bromophenyl)amide 4-hydrazide (compound VII)
[0069] The product VI (38g, 13mmol) was dissolved in 150ml of ethanol, hydrazine hydrate (0.72g, 144mmol) was added, and left at room temperature for one day, white crystals were precipitated, and 3.5g of product VII was obtained by filtration, with a yield of 87.72%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com