Paralichthys olivaceus male related Dmrt1 recombinant protein and application thereof
A technology of recombinant protein and flounder, applied in the field of molecular biology, can solve the problems of not allowed to use, unsuitable for application, high cost of use, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
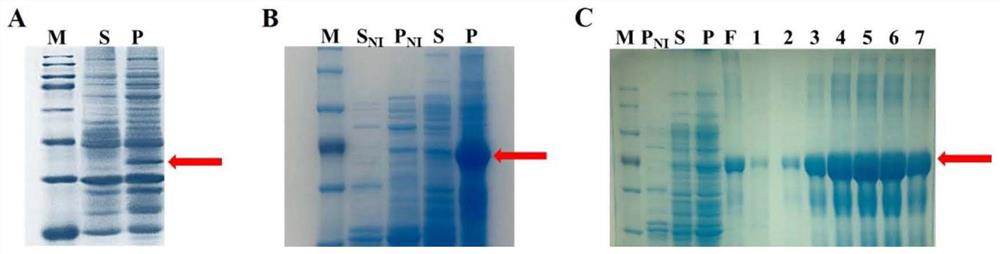
[0030] Example 1 Prokaryotic recombinant expression of flounder Dmrt1 protein
[0031] 1. Construction of recombinant plasmid Blunt E2-Dmrt1 and recombinant strain
[0032] Extracting the RNA of the flounder testis tissue, and reverse transcribing it into cDNA, using PCR to amplify the entire coding region of the flounder Dmrt1, the encoded amino acid sequence is shown in SEQ ID NO: 1, and then using the designed specific primers, its The sequence is:
[0033] Dmrt1-F: 5'-ATGAACAAGGACAAGCAGAG-3';
[0034] Dmrt1-R: 5'-ATTGATGCCATCCACGTCGA-3'
[0035] PCR amplification was carried out. After the amplified fragments were verified by gel recovery, purification and sequencing, they were connected to the linearized Blunt E2 vector. The recombinant plasmids were transformed into E. coli DH5α competent cells. PCR amplification detection, and handed over to Ruibo Xingke Biotechnology (Beijing) Co., Ltd. for sequencing identification.
[0036] Amplification conditions are:
[0037] P...
Embodiment 2
[0046] 1. Construction of recombinant plasmid pET-30a-EGFP-Dmrt1 and recombinant strain
[0047] Extracting the RNA of the flounder testis tissue, and reverse transcribing it into cDNA, using PCR to amplify the entire coding region of the flounder Dmrt1, the encoded amino acid sequence is shown in SEQ ID NO: 1, and then using the designed specific primers, its The sequence is:
[0048] Dmrt1-F: 5'-AAGGCCATGGCTGATATGAACAAGGACAAGCAGAG-3';
[0049] Dmrt1-R: 5'-TGAATCGATACGCATATTGATGCCATCCACGTCGA-3'
[0050] PCR amplification was performed. After the amplified fragment was verified by gel recovery, purification and sequencing, it underwent homologous recombination with the linearized pET-30a-EGFP vector. The recombinant plasmid was transformed into E. coli DH5α competent. The cloned colonies were detected by PCR amplification of the bacterial solution, and were handed over to Ruibo Xingke Biotechnology (Beijing) Co., Ltd. for sequencing and identification.
[0051] Amplificatio...
Embodiment 3D
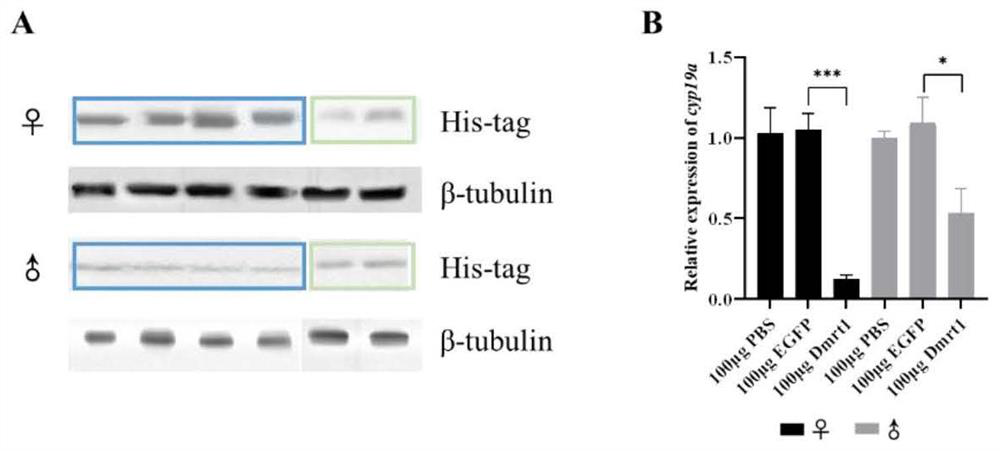
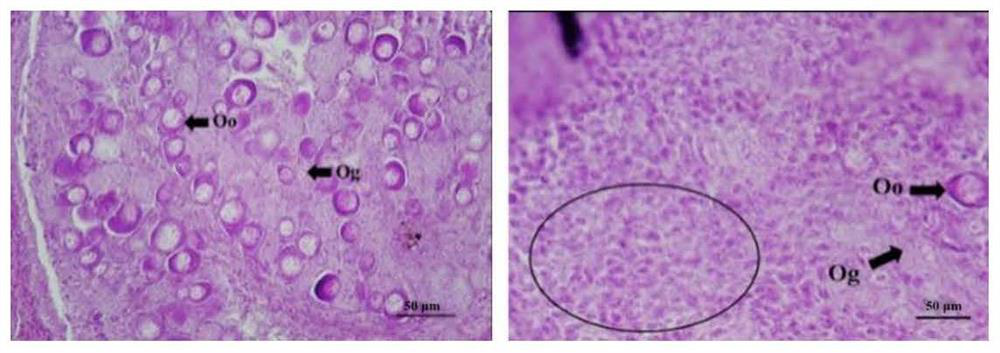
[0062] Example 3 In vitro activity verification of Dmrt1 recombinant protein
[0063] 1. Tissue incubation of Dmrt1 recombinant protein
[0064] The recombinant and purified flounder Dmrt1 protein in Example 2 was diluted to 1000 μg / mL in the aforementioned PBS.
[0065] Take the gonadal tissues of four healthy wild-type male and female adult fish, wash them repeatedly with the aforementioned PBS containing 1% penicillin (10kU / mL)-streptomycin (10mg / mL) mixed antibiotics, and cut them into about 1.0mm 3 The small pieces were placed in a 24-well plate, pre-incubated with L15 medium containing 1% antibiotics at 25 °C for 6 h, followed by Dmrt1 recombinant protein (100 μg) and Lipofectamine containing recombinant and purified Dmrt1 in Example 2. TM The fresh medium of 3000 transfection reagent was incubated at 25°C for 24h, and the incubated samples were stored at -80°C for later use.
[0066] 2. WB and qPCR analysis
[0067] A part of the samples were extracted with total pro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com