Difluoro-methane production process
A difluoromethane and production process technology, applied in the field of refrigerants, can solve the problems that the feed state of dichloromethane and the optimal feed temperature range are not given, and the feed temperature range of hydrogen fluoride is not given, so as to reduce the reaction The corrosion phenomenon of the device, the effect of improving the utilization rate of raw materials and product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
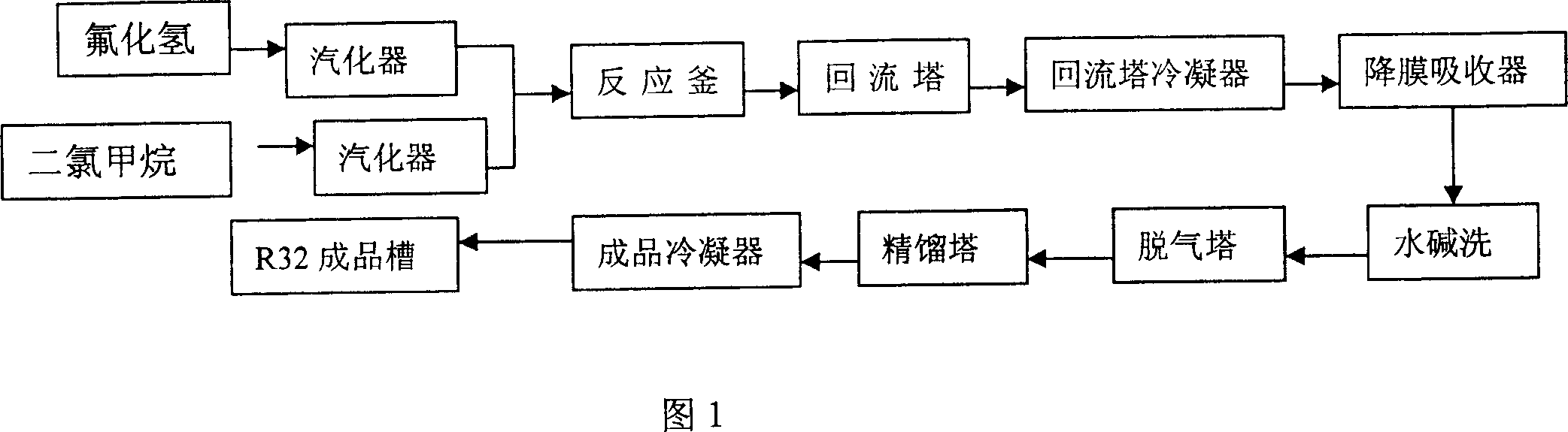
[0026] Hydrogen fluoride and dichloromethane are respectively pressed into the raw material vaporizer through a metering pump at a mass ratio of 1:2. Hydrogen fluoride vaporizes to a temperature of 100°C, and dichloromethane vaporizes to a temperature of 95°C. Then enter into the reactor equipped with fluorination catalyst to react. The reaction temperature is 95°C, and the reaction pressure is 1.2MPa. The crude difluoromethane produced by the reaction passes through the reflux tower and reflux condenser, and then the hydrogen chloride is removed by the falling film absorber, and then the pure difluoromethane is obtained after washing with water, alkali washing, degassing and rectification. After analysis, the purity of difluoromethane is ≥99.8%, and the utilization rate of raw materials is ≥95%. The reactor was not corroded during the implementation period.
Embodiment 2
[0028] Hydrogen fluoride and dichloromethane are respectively pressed into the raw material vaporizer through a metering pump at a mass ratio of 1:1.5. Hydrogen fluoride vaporizes to a temperature of 90°C, and dichloromethane vaporizes to a temperature of 95°C. Then enter into the reactor equipped with fluorination catalyst to react. The reaction temperature is 95°C, and the reaction pressure is 1.2MPa. The crude difluoromethane produced by the reaction passes through the reflux tower and reflux condenser, and then the hydrogen chloride is removed by the falling film absorber, and then the pure difluoromethane is obtained after washing with water, alkali washing, degassing and rectification. After analysis, the purity of difluoromethane is ≥99.8%, and the utilization rate of raw materials is ≥95%. The reactor was not corroded during the implementation period.
Embodiment 3
[0030] Hydrogen fluoride and dichloromethane are respectively pressed into the raw material vaporizer through a metering pump at a mass ratio of 1:2.5. Hydrogen fluoride vaporizes to a temperature of 100°C, and dichloromethane vaporizes to a temperature of 110°C. Then enter into the reactor equipped with fluorination catalyst to react. The reaction temperature is 95°C, and the reaction pressure is 1.2MPa. The crude difluoromethane produced by the reaction passes through the reflux tower and reflux condenser, and then the hydrogen chloride is removed by the falling film absorber, and then the pure difluoromethane is obtained after washing with water, alkali washing, degassing and rectification. After analysis, the purity of difluoromethane is ≥99.8%, and the utilization rate of raw materials is ≥95%. The reactor was not corroded during the implementation period.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com