Apoptosis signal-regulating kinase 1 inhibitors and methods of use thereof
a signal-regulating kinase and kinase technology, applied in the field of compounds and pharmaceutical compositions useful as ask1 inhibitors, can solve problems such as fat accumulation and fatty acid oxidation, and achieve the effect of prevention or treatmen
- Summary
- Abstract
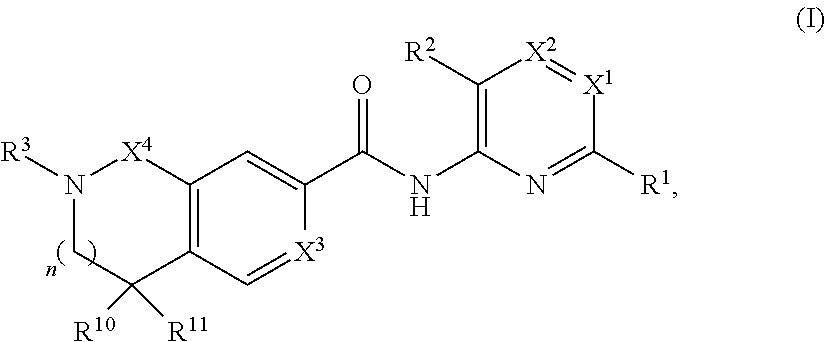
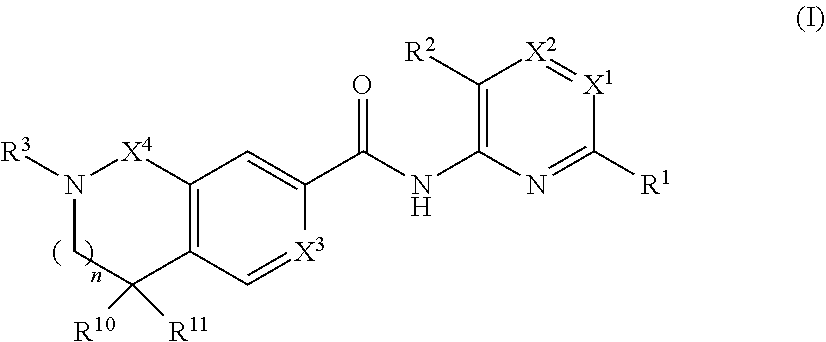
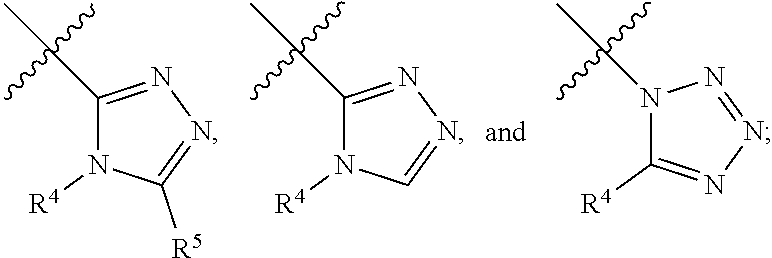
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
utyl)-5-fluoro-N-(6-(1-isopropyl-1H-tetrazol-5-yl)pyridin-2-yl)-2,3-dihydrobenzo[d]isothiazole-6-carboxamide 1,1-dioxide
[0260]
Step 1-1: Synthesis of N-isopropyl-6-nitropicolinamide
[0261]
[0262]Hunig's base (24.9 mL, 143 mmol, 2.0 eq) was added to a suspension of 6-nitropicolinic acid (1-6) (12.0 g, 71.4 mmol, 1.0 eq) in DCM (198 mL) and DMF (40 mL) at 0° C. Isopropylamine (1-7) (8 mL, 93 mmol, 1.3 eq) was added, followed by HATU (30 g, 79 mmol, 1.1 eq) and the reaction was stirred for 5 hrs at 0° C. The reaction was quenched with brine / water (300 mL) and diluted with DCM (200 mL). The layers were separated and the organic layer was washed with water (200 mL) and brine (200 mL). The organic layer was dried (MgSO4), filtered, and concentrated under reduced pressure. The resultant crude material was purified by column chromatography eluting with hexanes / EtOAc (0% EtOAc→40% EtOAc) to afford pure compound (1-8) (11.5 g, 55.0 mmol, 77%): 1H NMR (400 MHz, Chloroform-d) δ 8.58 (dd, J=7.7, 1....
examples 2 and 3
l)-5-fluoro-N-(6-(1-(1-hydroxypropan-2-yl)-1H-tetrazol-5-yl)pyridin-2-yl)-2,3-dihydrobenzo[d]isothiazole-6-carboxamide 1,1-dioxide and (R)-2-(tert-butyl)-N-(6-(1-(1-hydroxypropan-2-yl)-1H-tetrazol-5-yl)pyridin-2-yl)-5-methoxy-2,3-dihydrobenzo[d]isothiazole-6-carboxamide 1,1-dioxide
[0279]
Step 2-1: Synthesis of (R)-2-(6-nitropicolinamido)propyl acetate
[0280]
[0281]Hunig's base (3.1 mL, 5.3 mmol, 1.5 eq), a solution of amine (2-9) (1.1 g, 4.6 mmol, 1.3 eq) in DMF (5.0 mL), and HATU (2.0 g, 5.3 mmol, 1.5 eq) was added to a solution of 6-nitropicolinic acid (1-6) (592 mg, 3.5 mmol, 1.0 eq) in DMF (6.7 mL) and the reaction was stirred overnight. The reaction was quenched with H2O and diluted with EtOAc. The layers were separated and the aqueous layer was extracted with EtOAc (2×). The combined organic layers were washed with water, brine (2×), dried (MgSO4), filtered, and concentrated under reduced pressure. The resultant yellow gum was purified by column chromatography eluting with hexane...
example 4
utyl)-6-fluoro-N-(6-(1-isopropyl-1H-tetrazol-5-yl)pyridin-2-yl)-3,4-dihydro-2H-benzo[e][1,2]thiazine-7-carboxamide 1,1-dioxide
[0290]
Step 4-1: Synthesis of 5-bromo-N-(tert-butyl)-4-fluoro-2-formylbenzenesulfonamide
[0291]
[0292]Silver nitrate (377 mg, 2.22 mmol, 3.0 eq) was added to a suspension of compound (1-14) (357 mg, 0.74 mmol, 1.0 eq) in a mixture of THF (2.8 mL) and H2O (0.9 mL) and the reaction was heated at 80° C. overnight. The reaction was quenched with H2O and diluted with EtOAc. The layers were separated and the aqueous layer was extracted with EtOAc (2×). The combined organic layers were dried (MgSO4), filtered, and concentrated under reduced pressure. The resultant yellow solid was purified by column chromatography eluting with hexanes / EtOAc (0% EtOAc→25% EtOAc) to afford compound (4-9) as a white solid: 1H NMR (400 MHz, Chloroform-d) δ 10.42 (d, J=1.6 Hz, 1H), 8.37 (d, J=6.2 Hz, 1H), 7.70 (d, J=8.0 Hz, 1H), 5.20 (s, 1H), 1.26 (s, 9H); LCMS (ESI) m / z 336.0 (M−1).
Step 4-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com