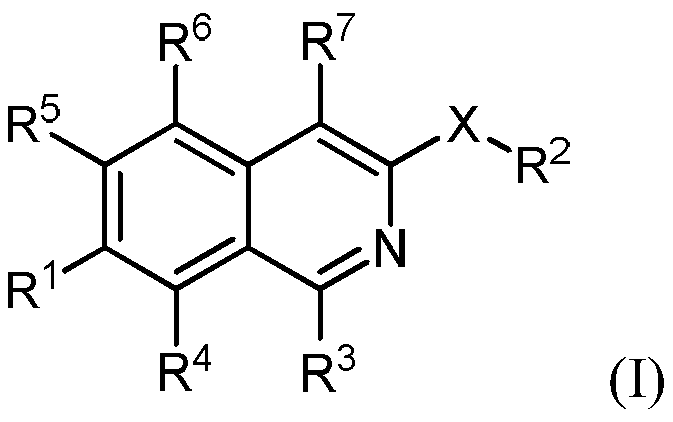
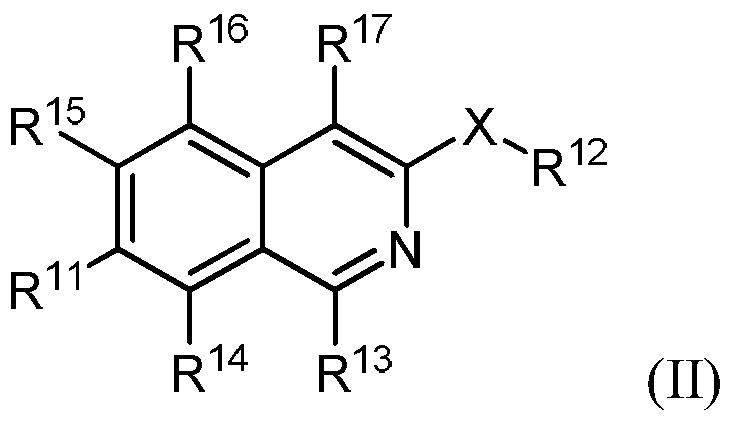
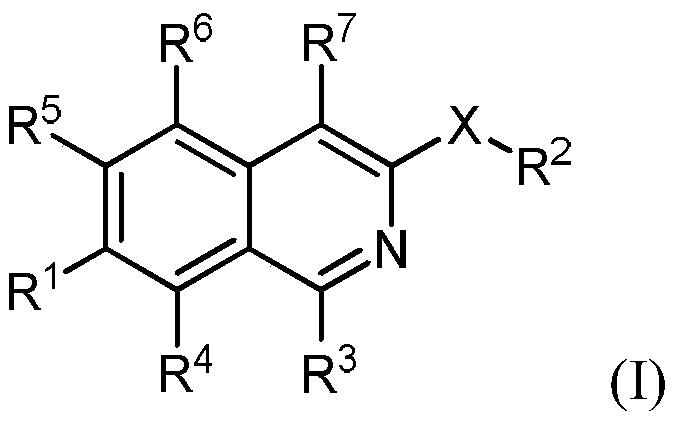
Isoquinoline derivatives as perk inhibitors
A technology for mammals and diseases, applied in the field of substituted isoquinoline derivatives, which can solve the problems of inability to synthesize important proteins, cell death, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0729] 5-(3-Benzylisoquinolin-7-yl)-7-methyl-7H-pyrrolo[2,3-d]pyrimidin-4-amine
[0730]
[0731]
[0732] Step 1: To a stirred solution of 2-iodobenzoic acid (10.0 g, 40.32 mmol, 1 equiv) in MeOH (100 mL) was added dropwise H at 0 °C 2 SO 4 (10 mL). The reaction mixture was warmed to 90 °C and stirred for 8 hours. The reaction mixture was cooled and concentrated. The residue was basified with saturated sodium bicarbonate at 0 °C and extracted with ethyl acetate (2 x 150 mL). The organic layer was washed with water and brine solution then dried over sodium sulfate and evaporated to give methyl 2-iodobenzoate as a colorless liquid (9.0 g, 85%).
[0733] 1 H NMR (400MHz, CDCl 3 )δppm 3.93(s, 3H), 7.15(t, J=8.0Hz, 1H), 7.40(t, J=7.2Hz, 1H), 7.80(d, J=8.0Hz, 1H), 7.99(d, J = 8.0Hz, 1H).
[0734] Step 2: To a stirred solution of methyl 2-iodobenzoate (5.0 g, 19.08 mmol, 1 equiv) and NBS (3.73 g, 20.99 mmol, 1.1 equiv) in acetic acid (10 mL) was dropped at 20-40 °C Ad...
Embodiment 2
[0741] 5-(3-(3,5-Dimethylbenzyl)isoquinolin-7-yl)-7-methyl-7H-pyrrolo[2,3-d]pyrimidin-4-amine
[0742]
[0743]
[0744] Step 1: To a stirred solution of 4-bromophthalic acid (9.0 g, 37.55 mmol, 1 equiv) in THF (90 mL) was added BH dropwise at 0 °C 3 .DMS (35 mL, 375 mmol, 10 equiv). The reaction mixture was warmed to room temperature and stirred overnight. The reaction mixture was cooled and quenched slowly with MeOH, then evaporated to give the crude product, which was purified by flash column chromatography on silica gel. Compound eluted in 1.5% MeOH:DCM. Fractions with product were evaporated to give (4-bromo-1,2-phenylene)dimethanol as a white solid (6.0 g, 75.9%).
[0745] 1 H NMR (400MHz, DMSO-d 6 )δppm 4.45(d, J=5.2Hz, 2H), 4.51(d, J=5.2Hz, 2H), 5.12(t, J=5.6Hz, 1H), 5.20(t, J=11.4Hz, 1H), 7.31 (d, J=8.0 Hz, 1H), 7.40 (t, J=8.0 Hz, 1H), 7.54 (s, 1H).
[0746] Step 2: A solution of oxalyl chloride (14.2 mL, 165 mmol, 6.0 equiv) in DCM (120 mL) was cooled ...
Embodiment 3
[0757] 5-(3-Benzyl-8-fluoroisoquinolin-7-yl)-7-methyl-7H-pyrrolo[2,3-d]pyrimidin-4-amine
[0758]
[0759] Step 1: To a stirred solution of 1-bromo-2-fluoro-4-iodobenzene (5.0 g, 16.66 mmol, 1 equiv) in THF (50 mL) was added dropwise LDA (8.3 mL, 16.66 mmol, 1.0 equivalent). The reaction mixture was stirred for 1 h, then dry ice was added portionwise at -78 °C. The reaction mixture was warmed and stirred overnight at room temperature. The reaction mixture was quenched with 1N HCl and extracted with 5% MeOH in DCM (3 x 60 mL). The organic layer was dried over sodium sulfate, filtered and concentrated to give 3-bromo-2-fluoro-6-iodobenzoic acid (3.5 g, 61.4%) as a brown solid. LCMS (ES) m / z = 344.0, 346.0 [M+H] + . 1 H NMR (400MHz, DMSO-d 6 ) δppm 7.53 (t, J = 8.4 Hz, 1H), 7.65 (d, J = 8.0 Hz, 1H), 14.18 (s, 1H).
[0760] Step 2: To a stirred solution of 3-bromo-2-fluoro-6-iodobenzoic acid (3.3 g, 9.59 mmol, 1 equiv) in DCM (50 mL) was added SOCl dropwise at 0 °C 2(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com