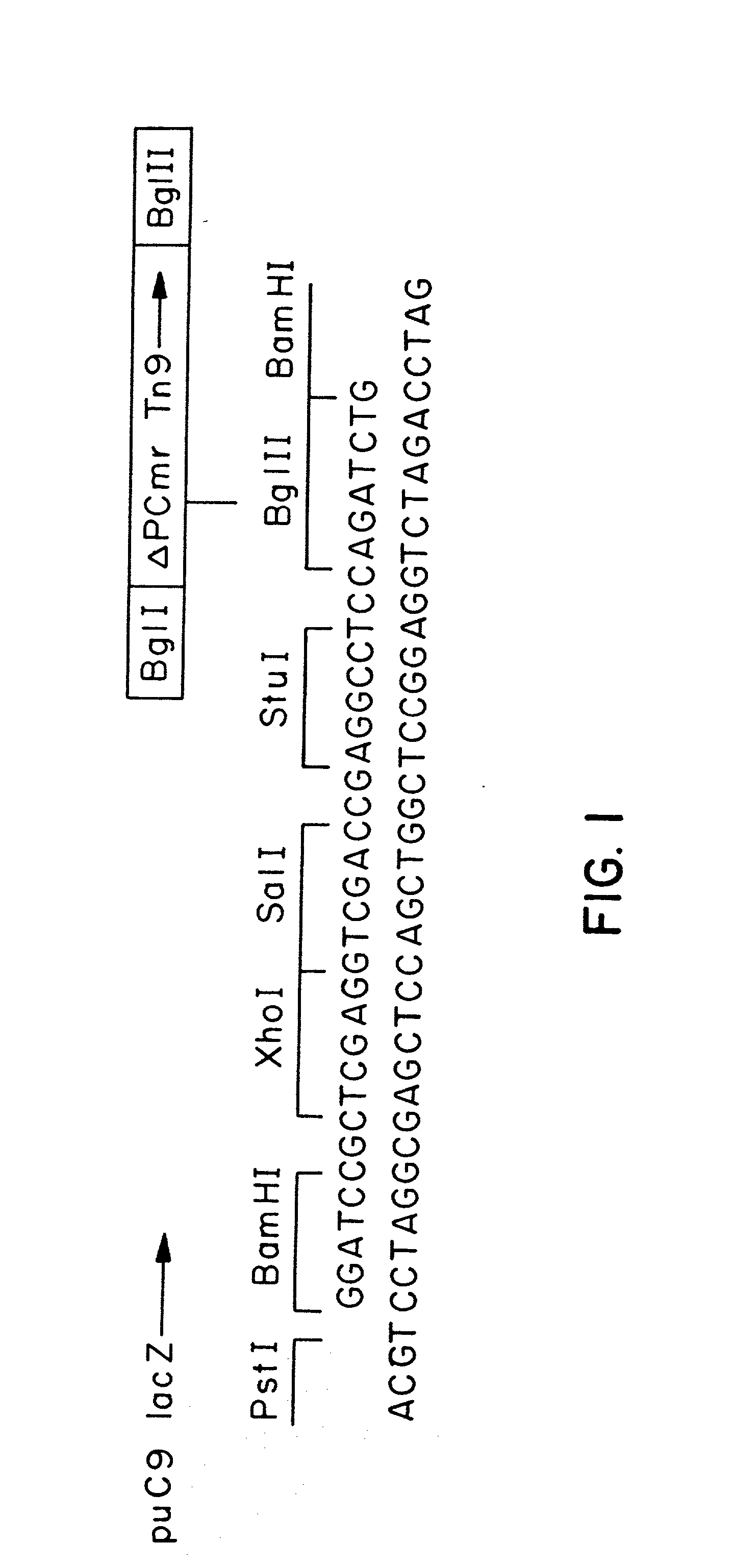
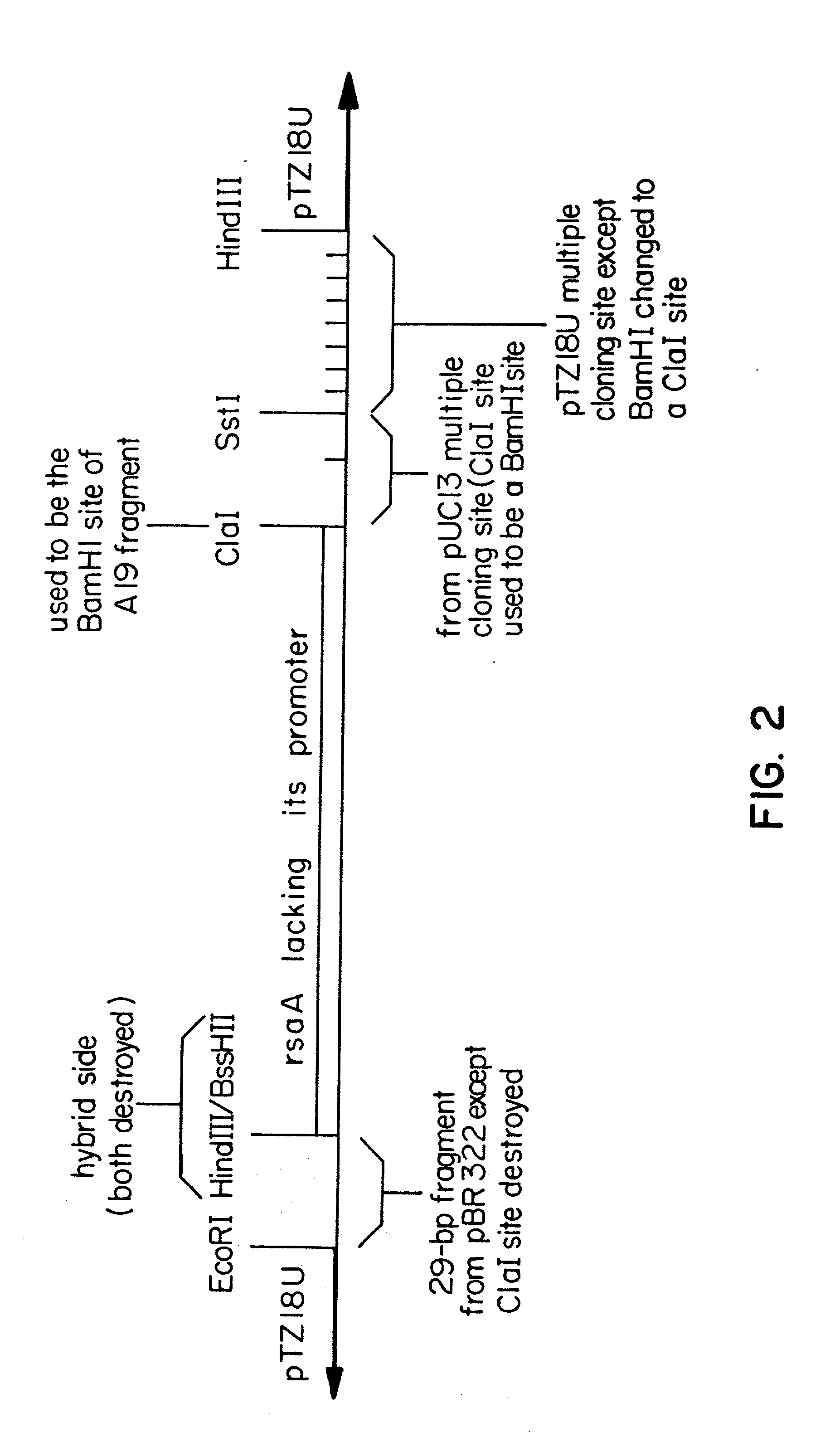
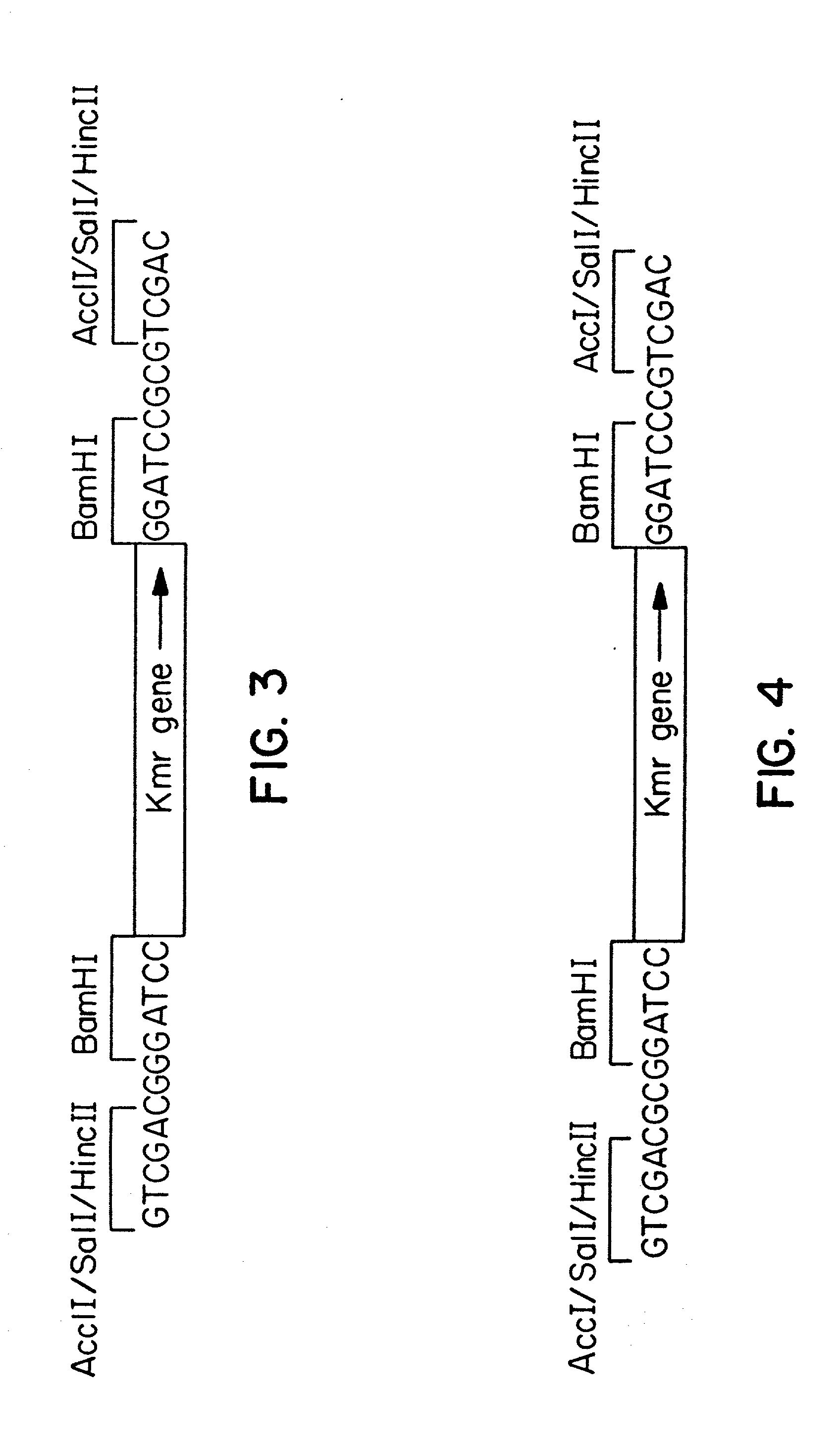
Expression and secretion of heterologous polypeptides from caulobacter
a technology of heterologous polypeptides and caulobacter, which is applied in the field of expression and secretion of heterologous polypeptides from caulobacter, can solve the problems of limited opportunities limited nutrient availability, and limited use of such surface proteins as a vehicle for expression and/or presentation of heterologous polypeptides, etc., to promote secretion and culture rapid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
Insertion of Cadmium Binding Polypeptides into Specific Sites
[0061] An insertion of the above described 12 bp linker was made at the TaqI site that corresponds to amino acid #188, frame #3 (see FIG. 6; SEQ ID NO:6; and, SEQ ID NO:7). This created a unique BamHI site at that position. Because the precise position of the TaqI site could be assessed from the DNA sequence information available for the rsaA gene, the necessary translation frame was known and thus a single construction of a metallothionein gene was made. This was done by excision of the coding sequence of monkey metallothionein II peptide (60 amino acids comprising 10 cysteine residues and having a molecular weight of about 5000) at known restriction sites and adapting the gene ends with BamHI linkers with appropriate base pair spacers for the needed translation frame.
[0062] After insertion into the BamHI site created at position 188, frame 3, several clones were examined by determining whether they could bind elevated le...
example 3
Investigation of Other Permissive Sites in rsaA Gene
[0064] A library of 240 BamHI linker insertions was created using the procedures of Example 1. Of the 240 insertions, 45 target sites in the rsaA gene were made with TaqI. 34 of the latter insertions were discarded because the clones contained deletions of rsaA DNA as well as the linker insertions. The remaining 11 resulted in 5 non-permissive and the 6 permissive sites described in Example 1. The remaining 195 insertions in the library were made using the enzymes HinPI, AciI, and MspI to create target sites as outlined in Example 1. Of the latter 195 insertions, 49 permissive sites were located for a total of 55. Of those sites scored as non-permissive, some may have had deletions of rsaA DNA at the linker insertion site. One BamHI linker insertion at a TaqI site thought to be permissive was later found by nucleotide sequencing to be located outside the rsaA structural gene reducing the total number of permissive sites to 54 from ...
example 4
Further Studies with Cadmium Binding Polypeptides
[0066] The results described for Example 3 indicated that it would be possible to insert metallothionein at multiple places in the rsaA protein and thereby enhance the metal binding capacity of such a transformed Caulobacter. However, when the procedures of Example 2 were repeated to insert the metallothionein coding sequence into others of the 54 permissive sites identified in the preceding Example in each case, the transformed Caulobacter did not secrete a chimeric protein and did not synthesize an S-layer. Furthermore, the transformed Caulobacter of Example 2 was stable as long as the transformants were frozen immediately after isolation. When continuously cultured for approximately one week, the transformants deleted the metallothionein portion of the S-layer and the S-layer protein returns to its normal size.
[0067] Consideration of the predicted amino acid sequence of the rsaA protein shows that the latter protein lacks cysteine ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| adhesive | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com