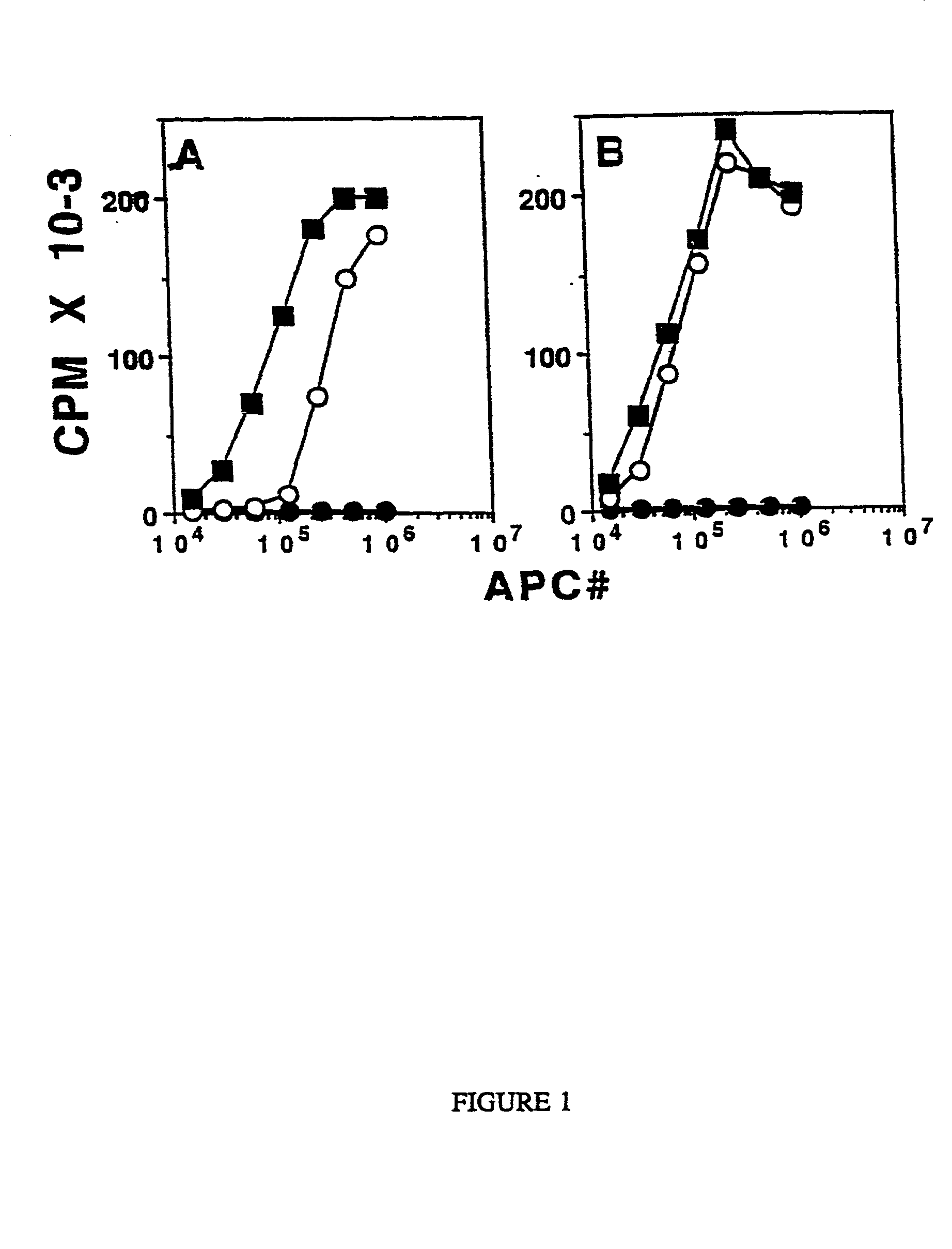
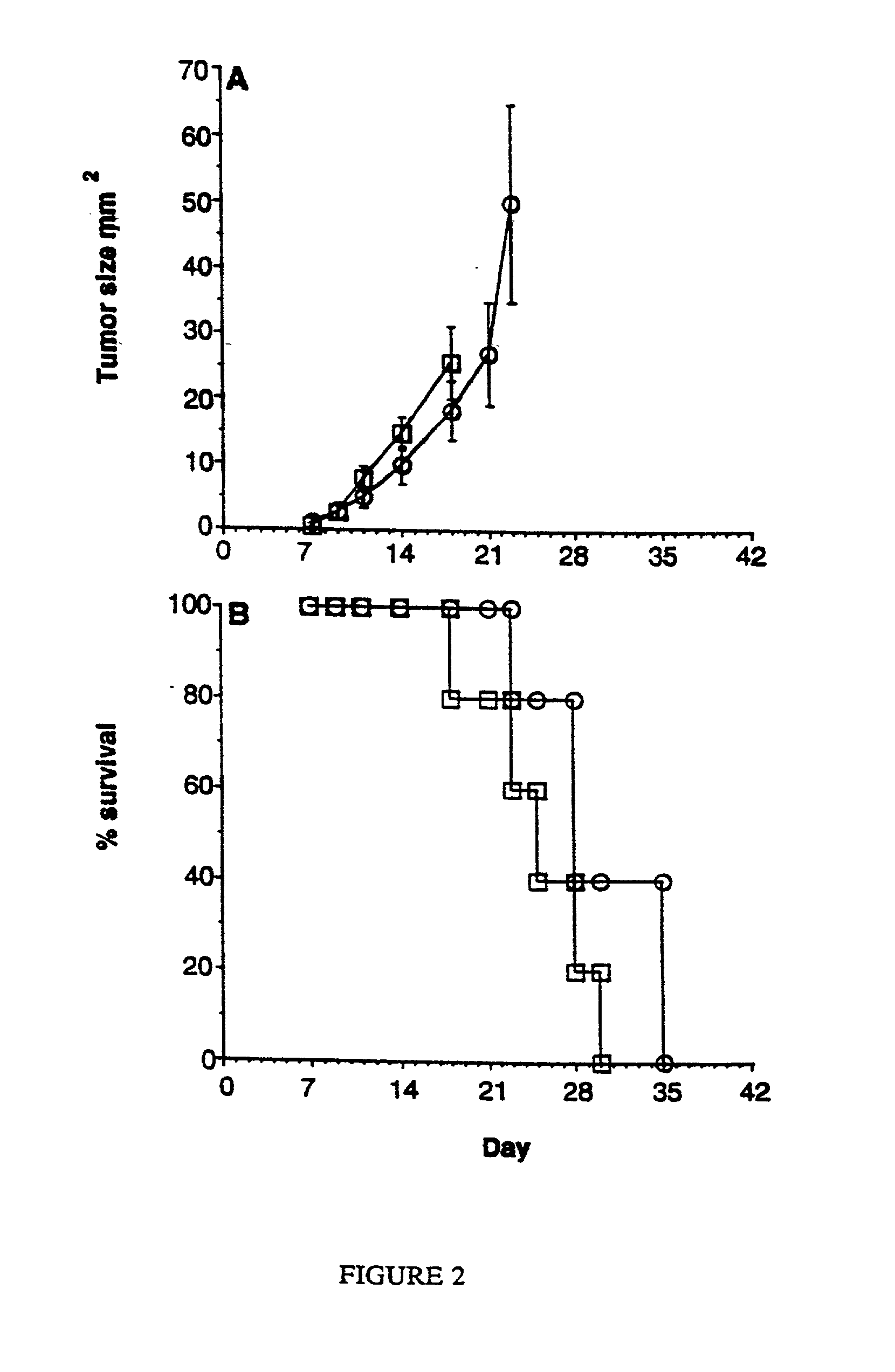
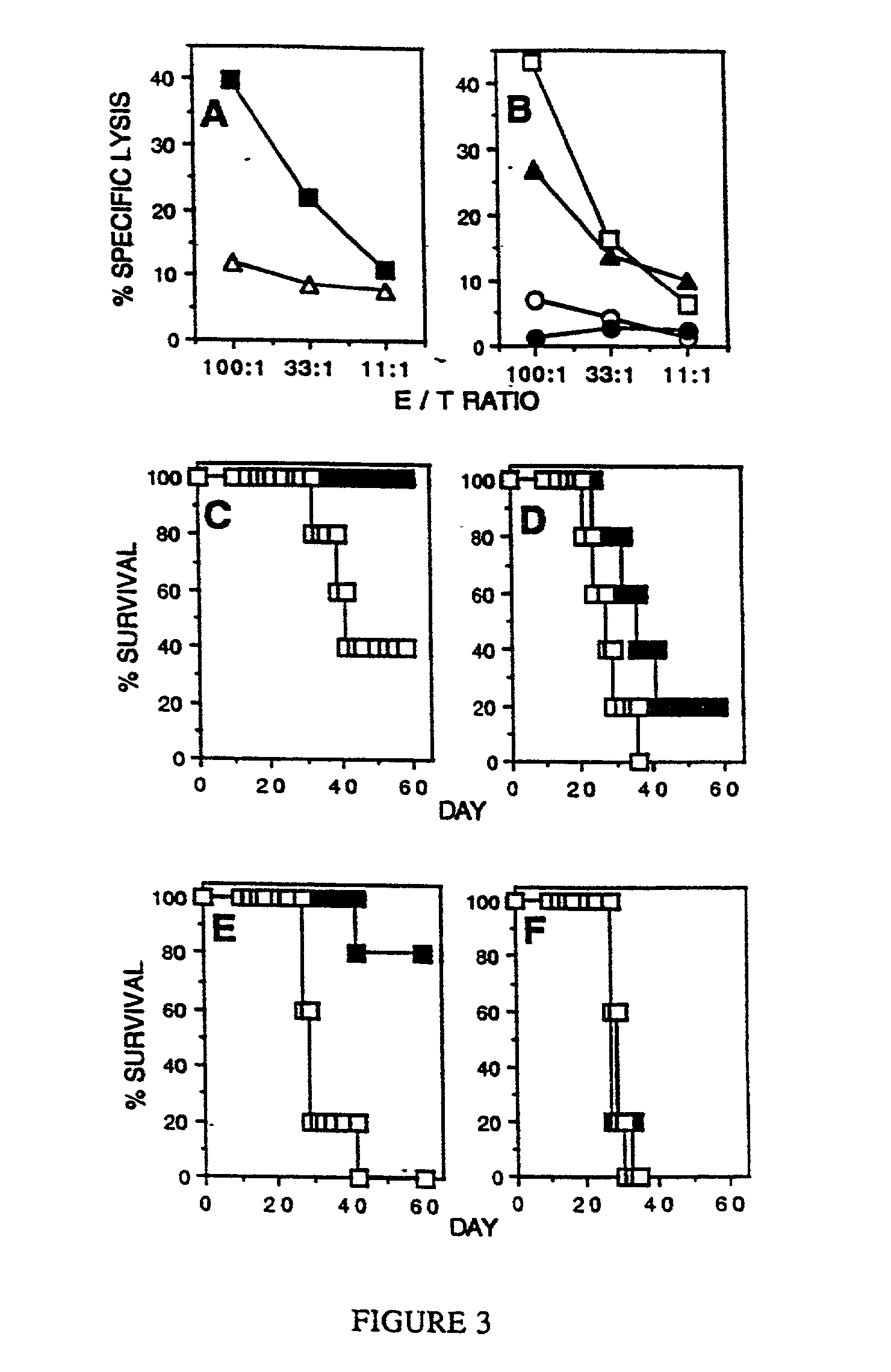
Stimulation of cell-mediated immune responses by targeted particulate genetic immunization
a technology of targeted particulate genetic immunization and cell-mediated immune response, which is applied in the direction of dna/rna vaccination, antibody medical ingredients, biocide, etc., can solve the problems of inability to disclose the targeting of genetic material to apcs for genetic immunization, inability to induce antigen-specific ctl responses in vivo, and inability to disclose the targeting of genetic material to apcs for genetic im
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0048] As used herein, the term "mammalian host" includes members of the animal kingdom, including but not limited to human beings.
[0049] As used herein, the term "DNA fragment" may include any nucleotide sequence, either DNA or RNA, which contains appropriate coding region and regulatory sequences to result in target cell expression of an antigenic protein or antigenic protein fragment for cell membrane presentation via the endogenous MHC Class I pathway.
[0050] As used herein, the term "particulate polynucleotide" may refer to a particulate made from materials including but not limited to gold, iron, and synthetic plastics wherein the particle comprises a population of DNA fragments as defined in the preceding paragraph.
[0051] The present invention relates to therapeutic or prophylactic genetic immunization of a mammalian host which comprises delivery of a DNA fragment to a target cell within the mammalian host, expression of the DNA fragment within the target cell, and subsequent ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| discharge pressure | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| adhesion | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com