Process for electrochemical decomposition of superalloys
a technology of electrochemical decomposition and superalloys, which is applied in the direction of electrolysis components, alkali metal halides, and normal temperature solutions, etc., can solve the problems of high value loss, difficult recycling, and inability to recycle economically,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
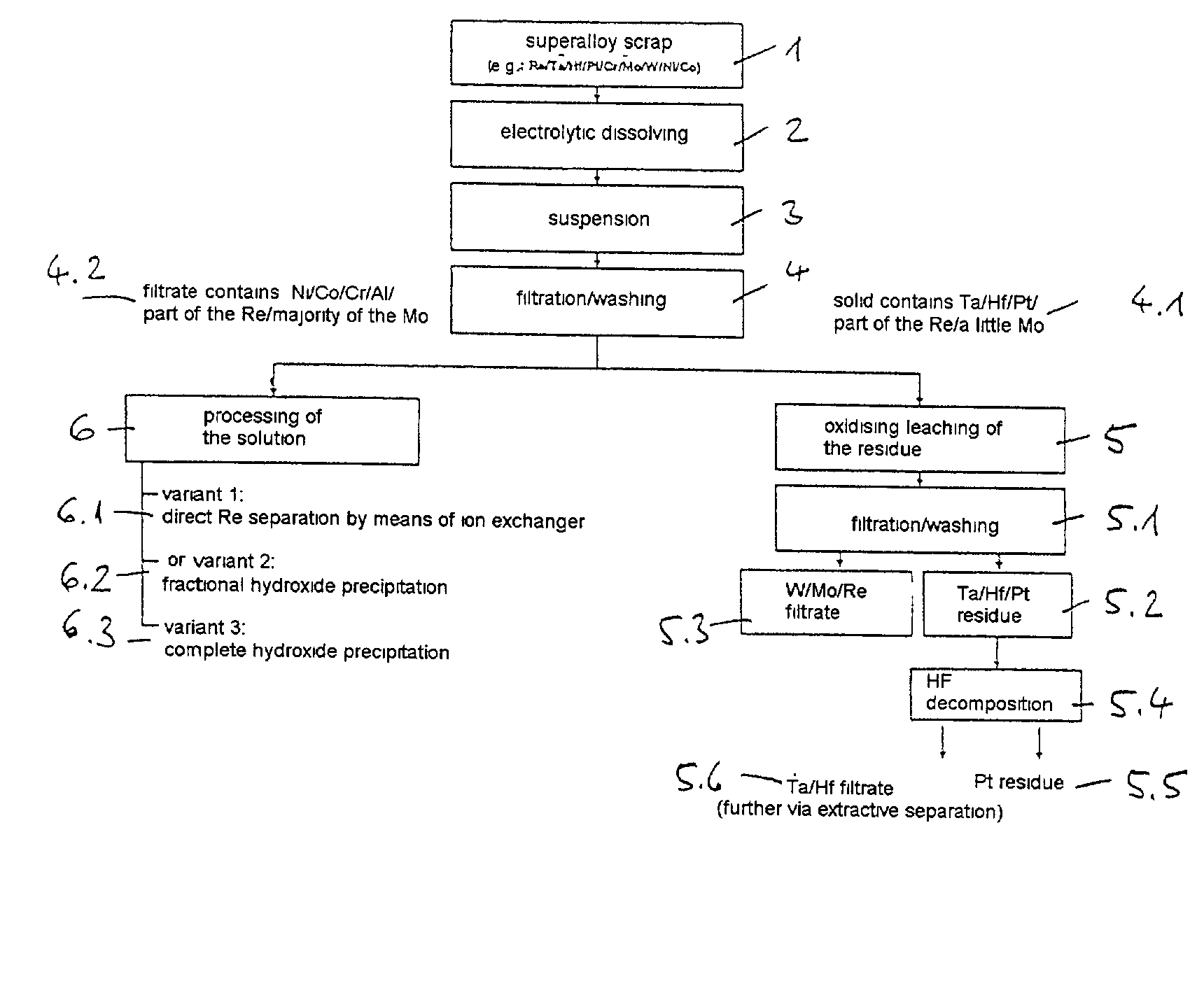
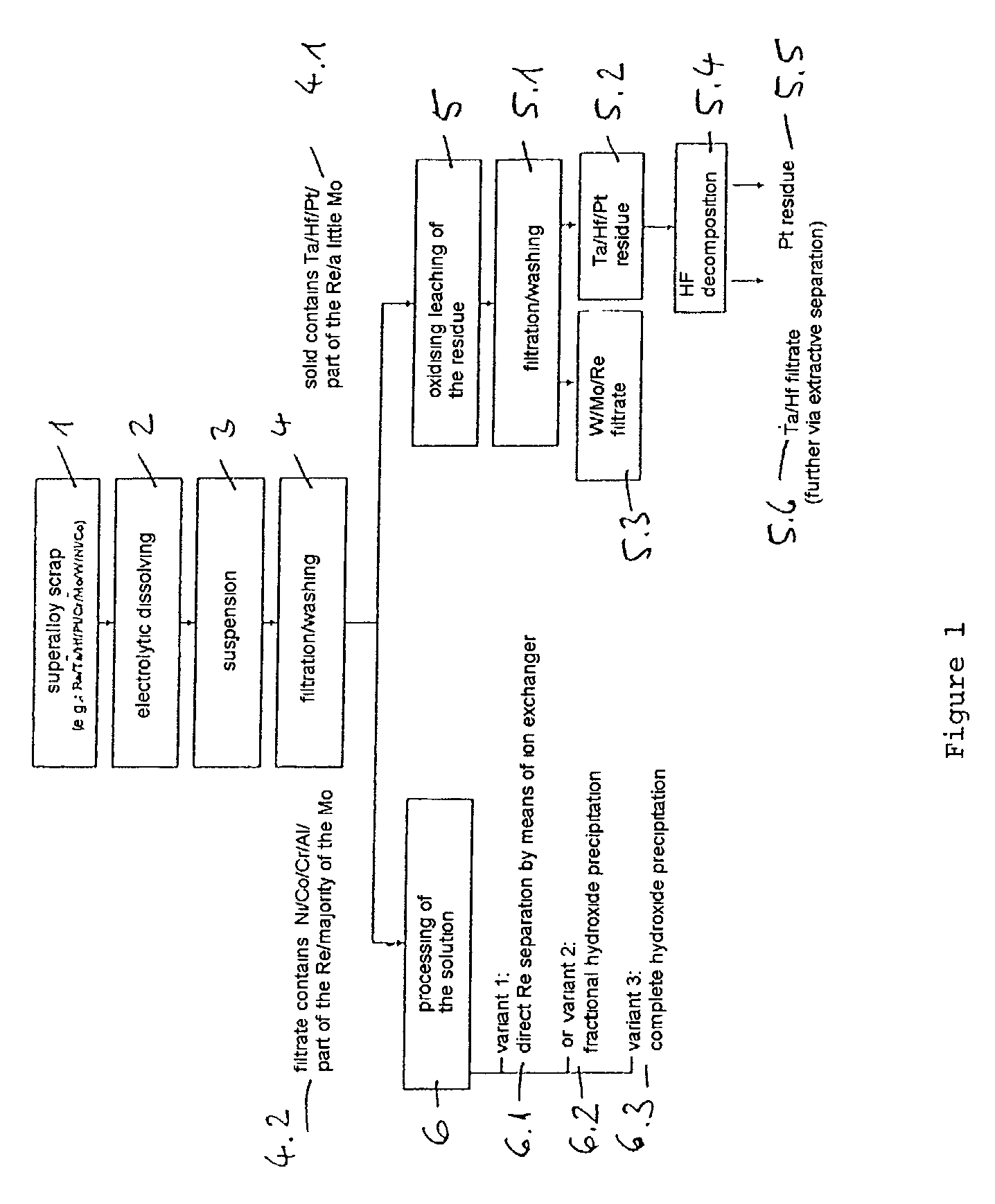
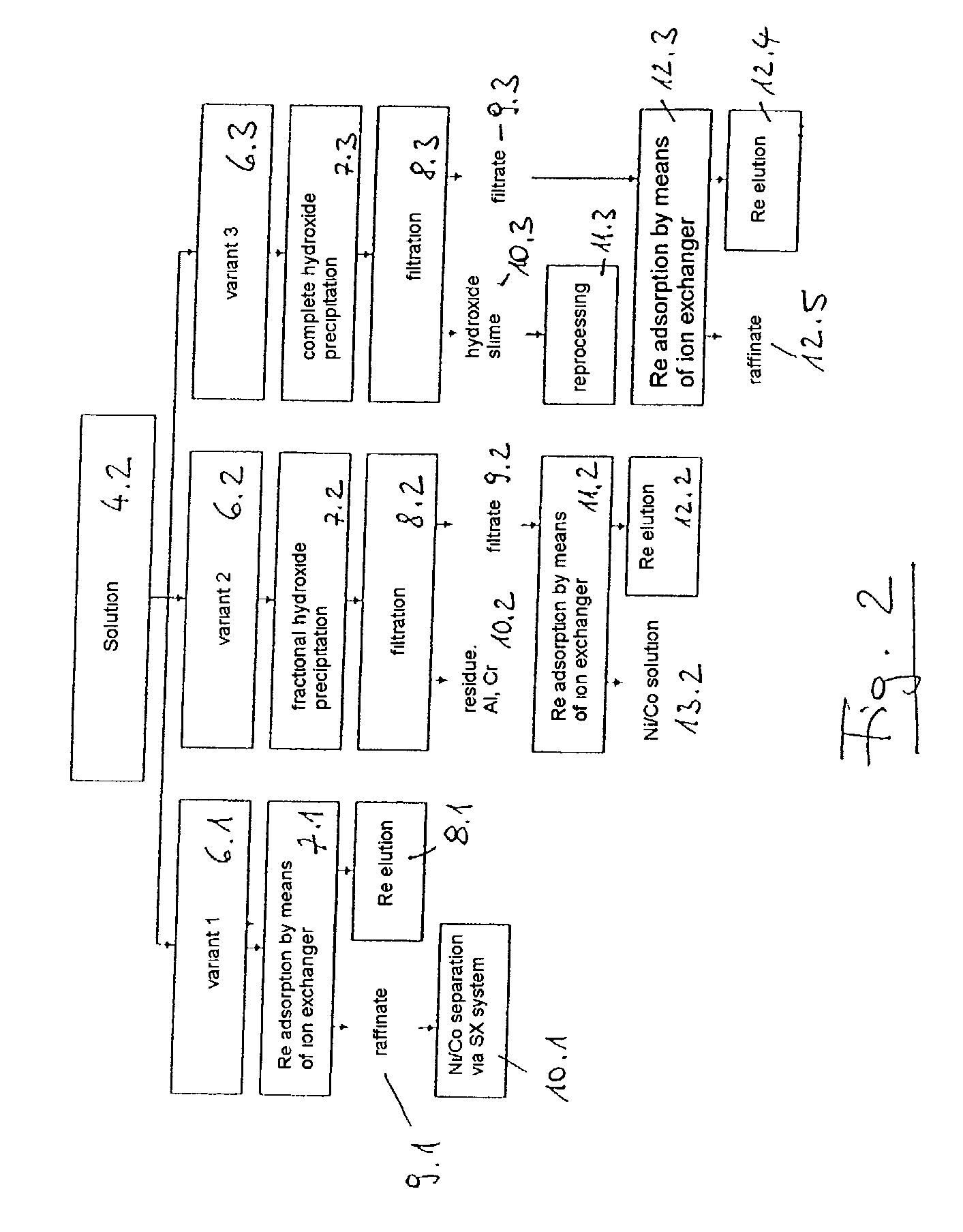
[0024] 10.4 kg of dilute hydrochloric acid solution (18.5 wt. %) are placed in a 15-litre electrolysis cell made of polypropylene. Two titanium baskets filled with superalloy scrap, with a total scrap content of 8.0 kg (composition, wt. %: 8.5 Ta, 3.1 Re, 5.8 W, 9.8 Co, 60.9 Ni, 4.9 Cr, 5.1 Al, 1.9 Mo) are used as the electrodes. The electrode spacing is approximately 2 cm. The electrolytic dissolving is carried out at 70.degree. C. by means of a square-wave current at a frequency of 0.5 Hz, a current of 50 amperes and a resulting voltage of approximately 3 to 4 volts. After an electrolysis time of 25 hours, the amount of scrap detached or dissolved is 1.6 kg. The resulting suspension is filtered and the residue (1) is washed with 0.63 kg of fully deionised water.
[0025] The 0.422 kg of filtration residue (1) contains wt. %: 39.5 Ta.sub.2O.sub.5, 6.2 ReO.sub.2, 27.8 WO.sub.3, 1.6 MoO.sub.3 and 25 H.sub.2O. The filtrate is purified with the wash water and wt. %: 0.3 HReO.sub.4, 0.4 H....
PUM
| Property | Measurement | Unit |
|---|---|---|
| frequency | aaaaa | aaaaa |
| electrolysis voltage | aaaaa | aaaaa |
| current frequency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com