Controlled release formulations containing an active ingredient, preferably melatonin, and the method of preparation
a technology of controlled release and active ingredients, which is applied in the direction of medical preparations, nervous disorders, endocrine system disorders, etc., can solve the problems of high cost of controlled release formulations (or pulsatile forms) developed recently, subsequent loss of sleep induction effect,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 2
Test in vitro for the Release Times of the Active Ingredients
[0059] The ready tablets, both in the pilot tests and in the experimental tests, as described above, were subjected to the following physical-chemical tests to obtain the following data:
[0060] a) weight uniformity;
[0061] b) hardness;
[0062] c) friability;
[0063] d) average content of melatonin;
[0064] e) content uniformity;
[0065] f) dissagregation;
[0066] g) time for the release of the active ingredient.
[0067] Weight uniformity, hardness, friability and disaggregation were checked by means of routine analyses according to standard procedures in the field. The average melatonin content and uniformity of content were determined by determination of the quantity of melatonin in the tablets.
Melatonin Analysis
[0068] The pilot batch samples were analysed for their melatonin content by means of direct radioimmunoassay method (RIA), using an anti-melatonin antibody (Stockgrand LTD) and 2-.sup.(125.vertline.)iodomelatonin (Amersham Inte...
example 3
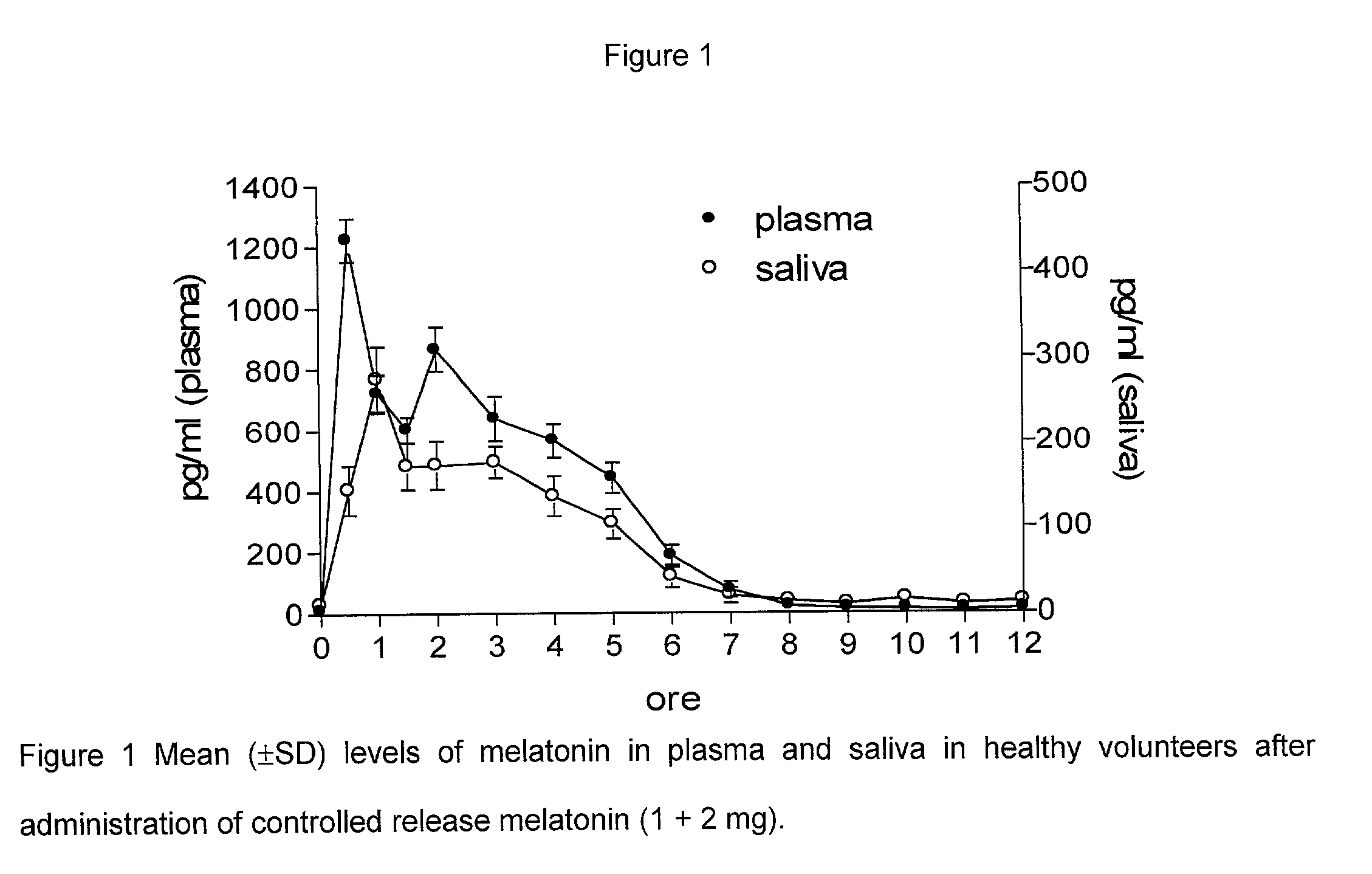
Pharmacokinetics in Man (Blood and Saliva: RIA)
Materials and Methods
[0078] Subjects
[0079] Six adult subjects, 4 males and 2 females with an average age of 35 took part in the test.
[0080] The volunteers had previously been informed of the purpose of the research and received a memorandum on how it would take place. All gave verbal consent. All were healthy and had never suffered from any chronic diseases; none of them had taken any medicines for the 2 weeks prior to the test nor during the period of research.
[0081] The subjects taking part in the test took one tablet of melatonin (2 mg+1 mg) at 8.45 a.m., in two groups of three subjects. The time of administration was chosen in so as not to interfere with the normal rhythm of melatonin secretion in the 12 hours afterwards. All the volunteers were exposed to light of an intensity of >2000 Lux during the experiment.
[0082] In the first stage the determination of melatonin pharmacokinetics after oral administration was carried out by mea...
example 4
Effects on Sleep in Man
Materials and Methods
[0099] Subjects and Treatment
[0100] Ten patients (average age 56.+-.3.6 years) suffering from psychophysiological insomnia, were used in the study. All of were classified according to The International Classification of Sleep Disorders, Revised Edition, ASDA, 1997. All were informed and gave their verbal consent to take part in the study. All received case report forms to fill in the following parameters: Total Sleep Time (TST), Sleep Latency (SL), Wake After Sleep Onset (WASO), Number of Awakenings (NA).
[0101] The treatment was in "crossover" and lasted a week for every formulation. Prior / after (according to a random assignment) to the treatment with controlled release formulations containing melatonin according to this research (1+2 mg of melatonin), the subjects took standard formulations of melatonin in 3 mg tablets, followed by a washout period of three days. In both treatments the tablets were given at 22.30 hours. Then, according to...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentrations | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com