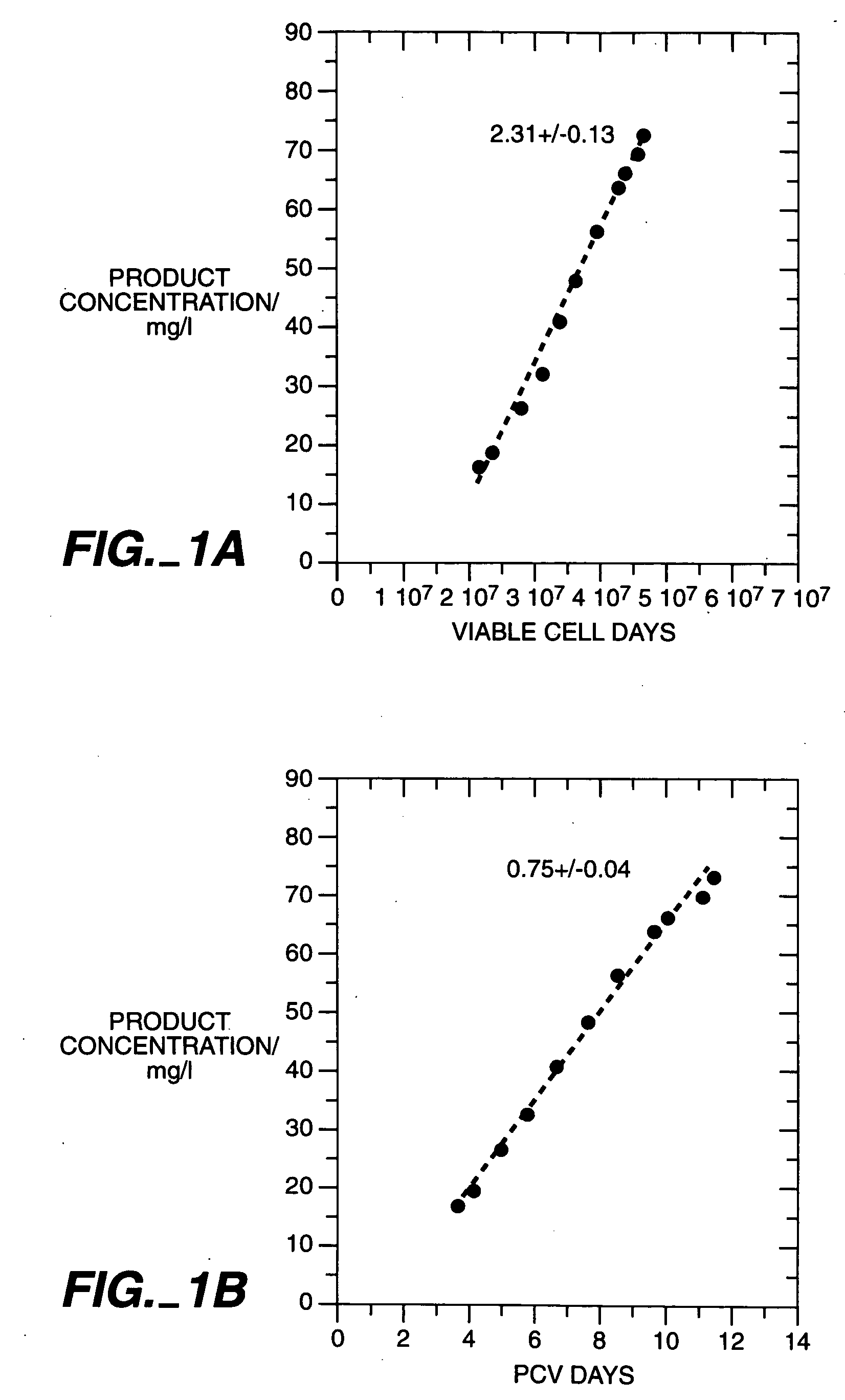
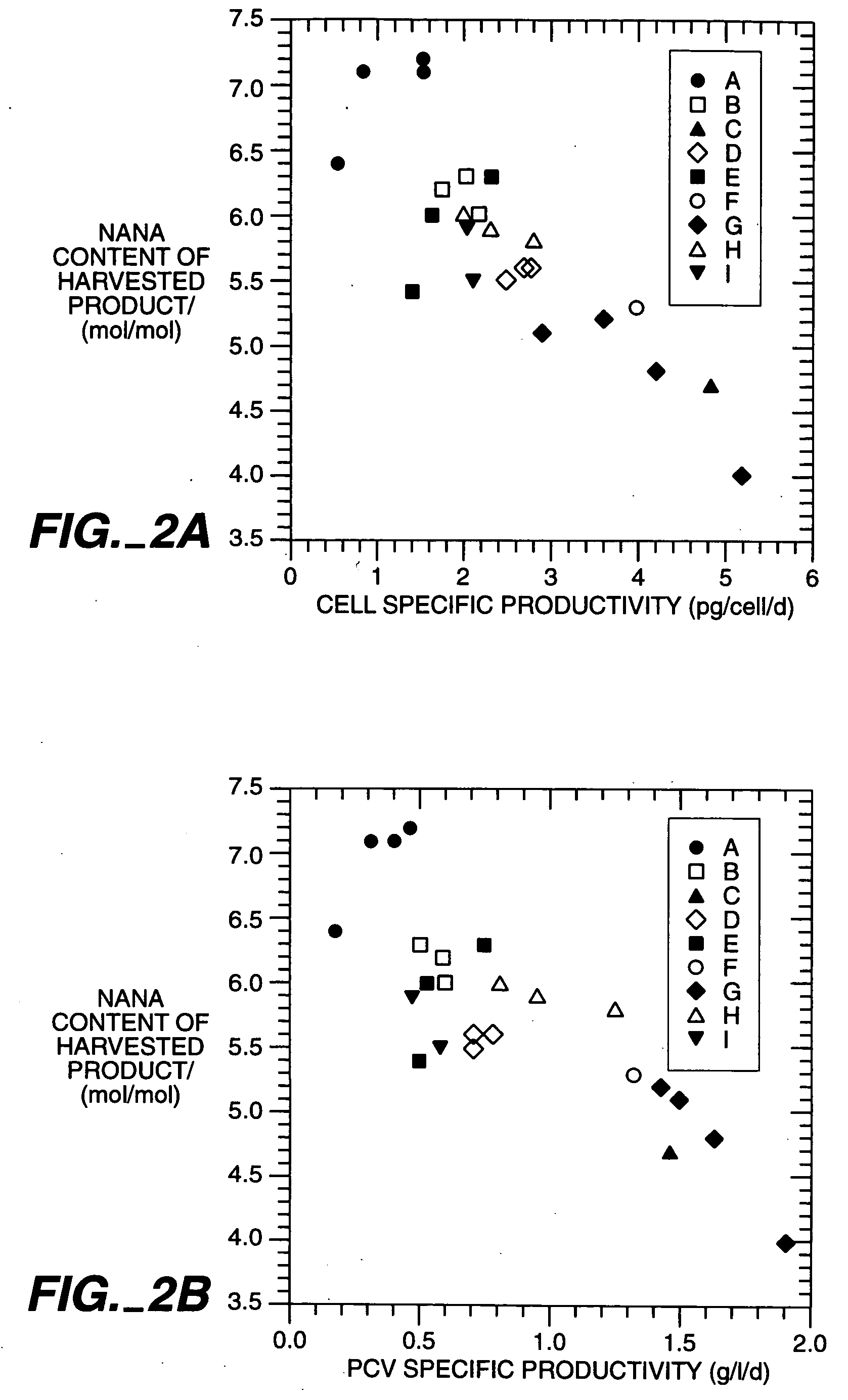
Mammalian cell culture process
a cell culture process and mammalian technology, applied in the field of mammalian cell culture process, can solve the problems of increasing the sialic acid content of mature proteins, little guidance in selecting appropriate concentrations of additives, and not addressing the effect of additives on protein glycosylation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0121] The biological effects of TNF-alpha and TNF-beta are mediated through specific receptors (Dembic et al., (1990) Cytokines, 2:231). Molecular cloning has demonstrated the existence of two distinct types of TNF receptors (TNFR) with apparent molecular masses of 55kD (type 1) (Schall et al., (1990) Cell 61:361) and 75 kD (type 2) (Smith et al., (1990) Science 248:1019), each of which naturally binds to both TNF alpha and TNF beta (Loetscher et al., (1990) Cell, 61:351; Shall et al. (1990) Cell, 61:361; Kohno et al., (1990) Proc. Natl. Acad. Sci. USA 87:8331). The extracellular portions of both receptors are found naturally as soluble TNF binding proteins (Kohno et al., supra). TNF agonists have been created which block the deleterious effect of TNF in various immune and inflammatory events (Peppel et al., (1991) J. Exp. Med., 174:1483-1489; Ulich (1993) Am. J. Path., 142:1335-1338; Howard, O. M. Z., (1993) Proc. Natl. Acad. Sci. USA 90:2335-2339; Wooley, P. H., (1993) J. Immunol...
example ii
[0144] Plasma pharmacokinetic half-lives and / or clearance rates of different preparations were determined. The preparations containing a higher sialic acid content in general exhibited increased plasma half-life and / or lower total clearance rates compared to preparations containing a decreased sialic acid content.
[0145] Methods
[0146] Seventeen male Sprague-Dawley-derived rats weighing 272-315 g were randomly assigned to one of three TNFR1-IgG.sub.1 fusion protein treatment groups (N=5 or 6 per group). The animals were injected intravenously with a nominal dose of 5 mg / kg of test material via a femoral vein cannula. The test materials chosen included two TNFR1-IgG.sub.1 preparations from a process in which the butyrate concentration during the production phase was 6 mM and the osmolality during the production phase was maintained at about 400 mOsm, Process I, and a third TNFR1-IgG.sub.1 preparation from a process in which the butyrate concentration was 12 mM and the osmolality during...
example iii
Monosaccaride Composition of TNFR1-IgG.sub.1
[0151] Determination of oligosaccharide carbohydrate composition and structure of TNFR1-IgG.sub.1, prepared as described in Example 1 showed that the sialic acid content of the various process version varied. Rapid plasma clearance was associated with high exposed GlcNAc, lower sialic acid on the oligosaccharide chains and (by inference) accessibility of the protein to mannose or galactose receptors. Slow plasma clearance was associated with more terminal sialic acid residues.
[0152] A. Sources of Test Material
[0153] TNFR1-IgG.sub.1 was produced according to the methods outlined as described in Example 1 above. Process I material was obtained from cell culture using 6 mM butyrate and an osmolality of about 400 mOsm during the production phase. Process material II was obtained from cell culture using 12 mM butyrate and an osmolality of about 500 mOsm during the production phase.
[0154] B. Methods
[0155] Release of intact neutral and amino-suga...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molar ratio | aaaaa | aaaaa |
| molar ratio | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com