In situ gelling drug delivery system
a drug delivery system and gel technology, applied in the direction of drug compositions, antibacterial agents, peptide/protein ingredients, etc., can solve the problems of low initial systemic concentration of active agents, and unsatisfactory traditional administration methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Evaluation of Solubility of PLGA in Organic Solvents
A sample of PLGA polymer was added to the indicated solvent and rotated overnight at room temperature, and the resulting mixture was examined for undissolved material. The results are shown in Table 1 below.
TABLE 1Solubility of PLGA polymers in selected organic solventsPolymerSolventVisual AppearancePLGA (70:30), 0.2 gPEG 400, 1 mlClear solutionPLGA (70:30), 0.2 gPEG 300, 2 mlClear solutionPLGA (70:30), 0.2 gPEG 200, 2 mlClear solutionPLGA (70:30), 0.1 gDMA, 20 DropsClear solutionPLGA (55:15), 0.1 gPEG 400, 1 mlPartially soluble*PLGA (50:50), 0.1 gPEG 400, 1 mlPartially soluble*PLGA (85:15), 0.1 gDMA, 1 mlClear solutionPLGA (50:50), 0.1 gDMA, 1 m1Clear solutionPLGA (90:10), 0.1 gPEG 400, 2 mlPartially soluble*PLGA (70:30), 0.1 gCremophor EL, 2 mlPartially solublePLGA (70:30), 0.1 gCremophor EL-P, 2 mlPartially solublePLGA (70:30), 0.1 gBenzyl alcohol, 0.5 mlClear solutionPLGA (70:30), 0.1 gBenzyl benzoate, 0.5 mlMiscible soluti...
example 2
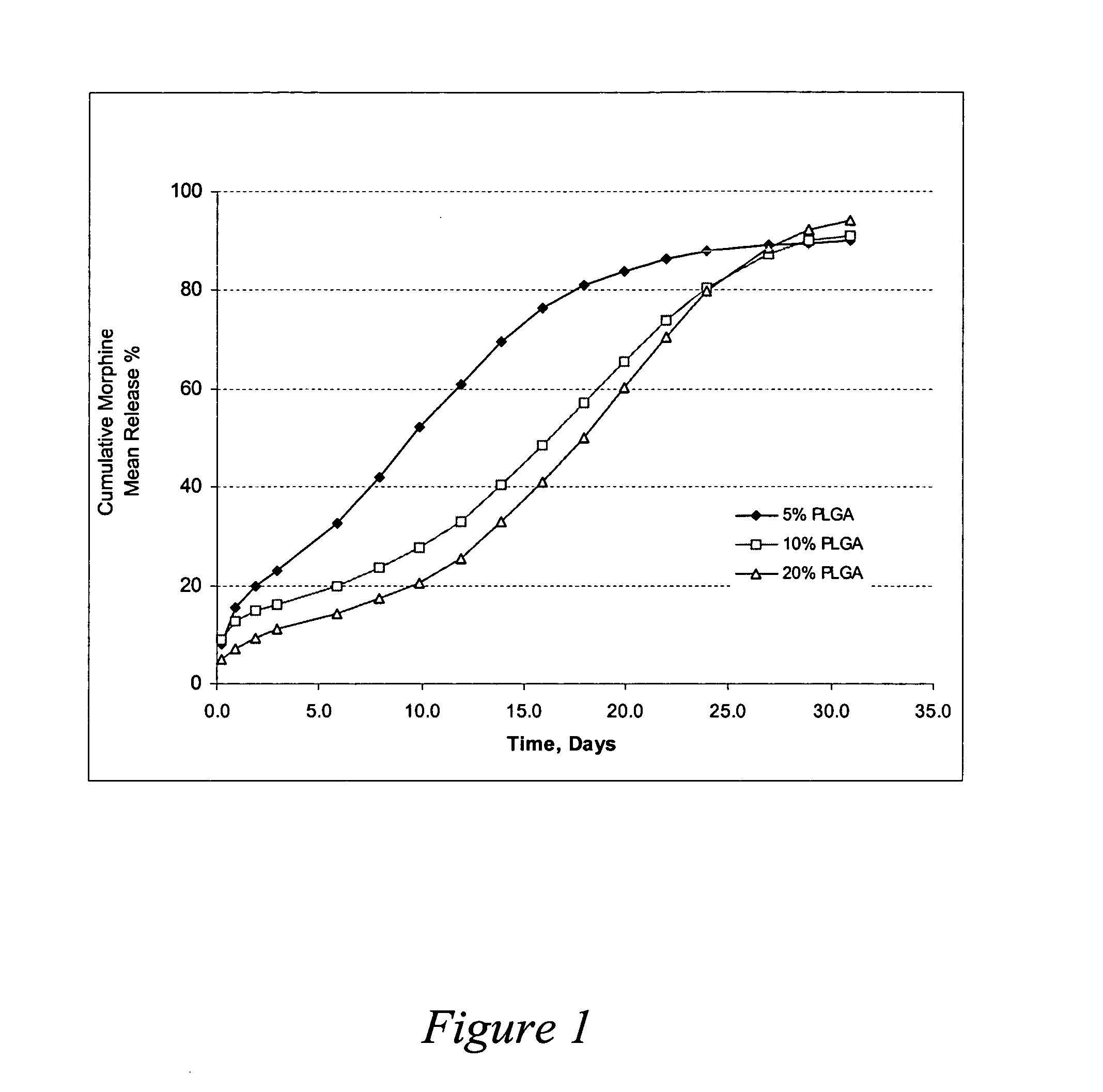
Release Profiles for Morphine-diclofenac Co-drug from PLGA (70:30) / PEG Formulations
Three formulations were evaluated to compare release profiles for morphine-diclofenac co-drug from different concentrations of PLGA (70:30): Formulation A was formulated at about 10 mg / ml morphine-diclofenac co-drug in PLGA (70:30) / PEG 400 solution (˜5% (w / v) PLGA in PEG). Formulation B was formulated at about 10 mg / ml morphine-diclofenac co-drug in PLGA (70:30) / PEG 400 solution (˜10% (w / v) PLGA in PEG). Formulation C was formulated at about 10 mg / ml morphine-diclofenac co-drug in PLGA (70:30) / PEG 400 solution (˜20% (w / v) PLGA in PEG).
Each formulation was loaded into a 1-ml syringe, and 100 μl aliquot was injected into a tube containing 10 ml of 10% plasma in HA (hyaluronic acid) phosphate buffer, pH 7.4. The samples were placed in a water bath at 37° C. for release study. At each time point, the entire release medium was removed and replaced with 10 ml fresh buffer. The removed solution was analy...
example 3
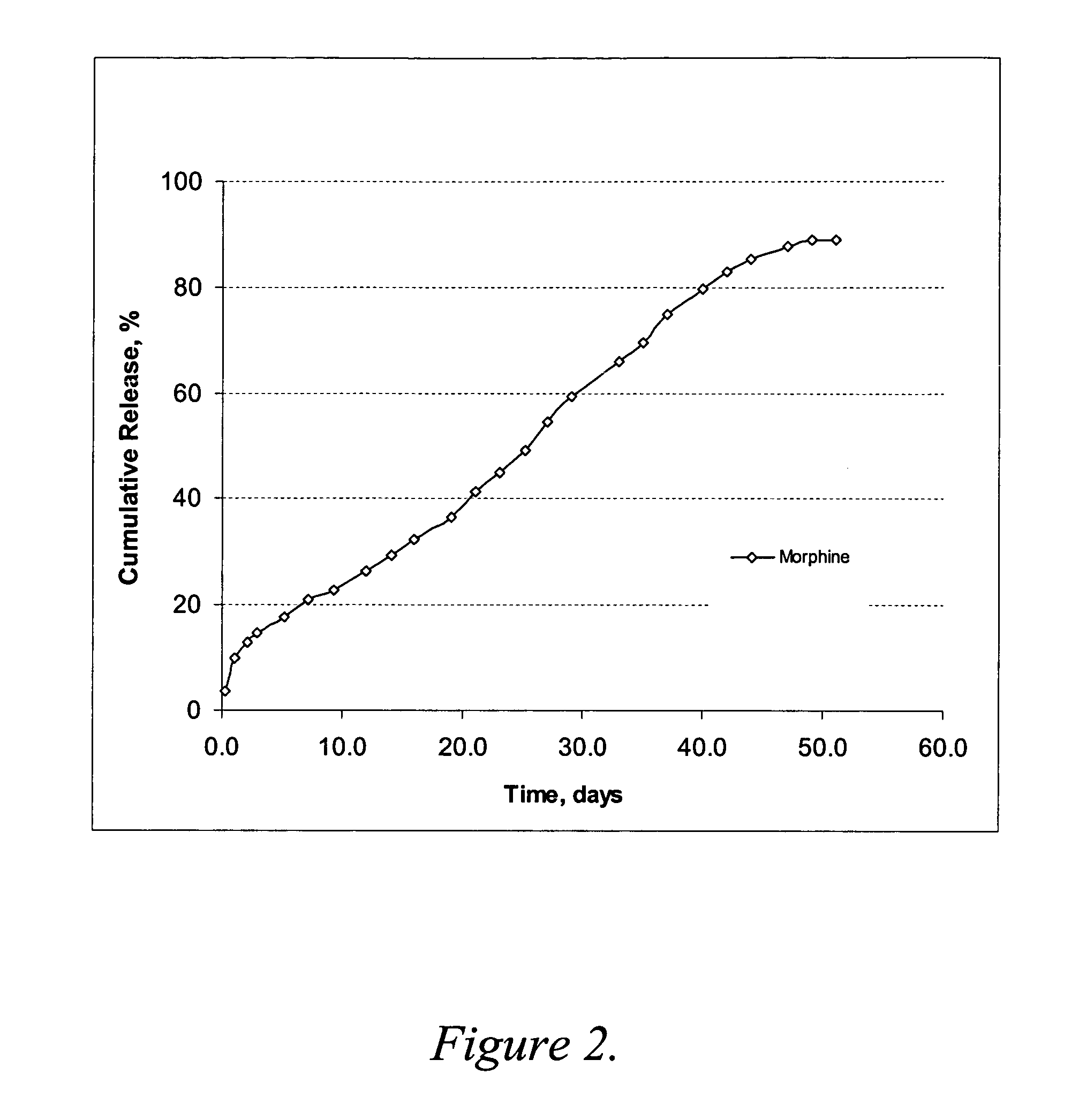
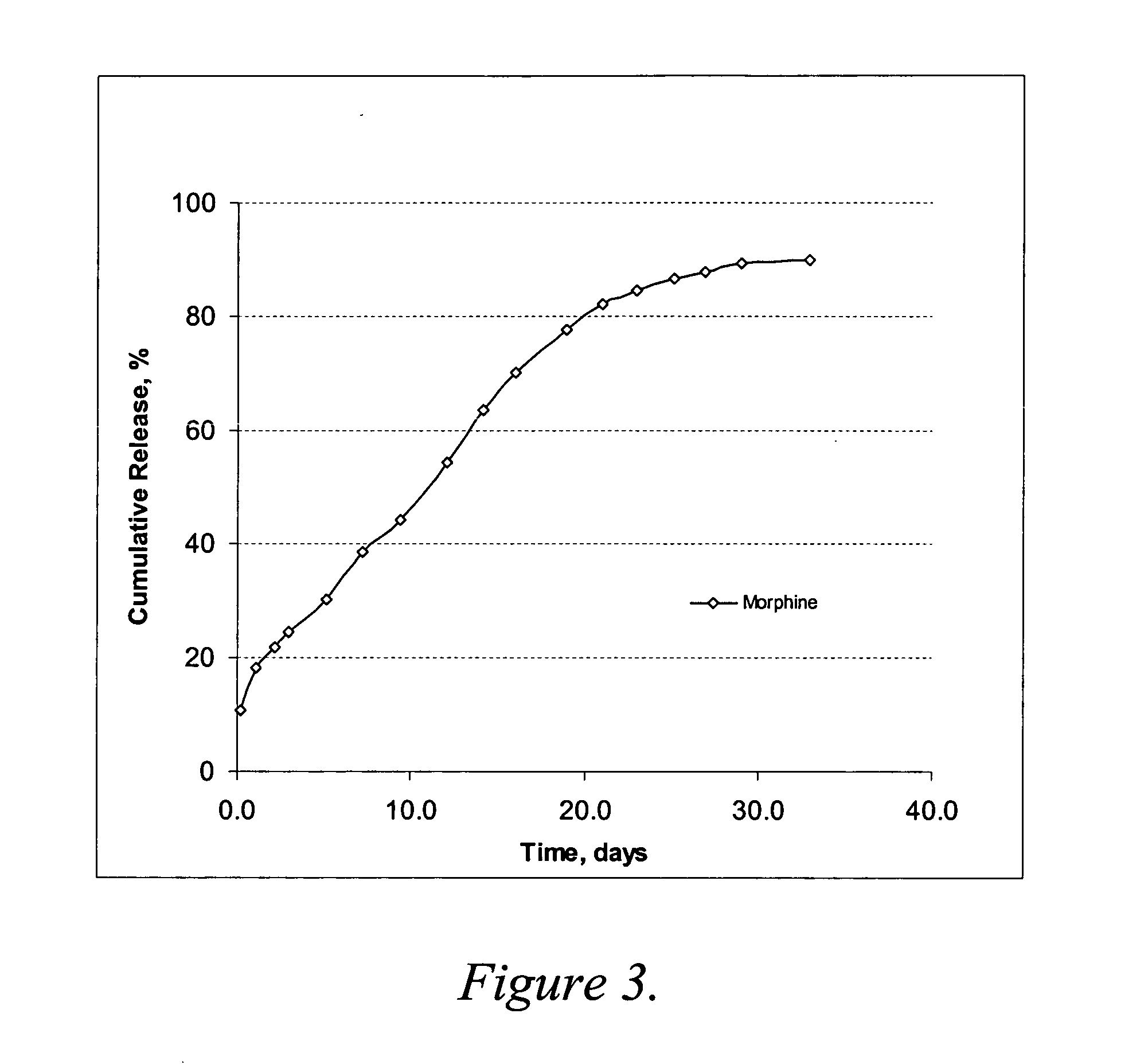
Release Rate Profile for Morphine-diclofenac Co-drug from PLGA (50:50) / PEG Formulation
The formulation was prepared with 12 mg / ml morphine-diclofenac co-drug in PLGA (50:50) / PEG 400 solution (˜5% (w / v) PLGA in PEG) and loaded into a 1-ml syringe, and 100 μl aliquot was injected into a test tube containing 10 ml of 10% plasma in HA (hyaluronic acid) phosphate buffer, pH 7.4. The samples were placed in a 37° C. water bath. At each time point, the entire release medium was removed and replaced with 10 ml fresh buffer. The removed solutions were analyzed for morphine, diclofenac and the co-drug contents by HPLC.
The results are shown graphically in FIG. 2. As compared to the results from Example 2, morphine release was much slower in this PLGA (50:50) formulation, even where the PLGA concentration was low as ˜5% (w / v). About 80% of the morphine was released over 40 days. It is most likely that the higher molecular weight of PLGA (50:50) reduces the release rate of morphine.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com