Nonaqueous electrolyte battery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
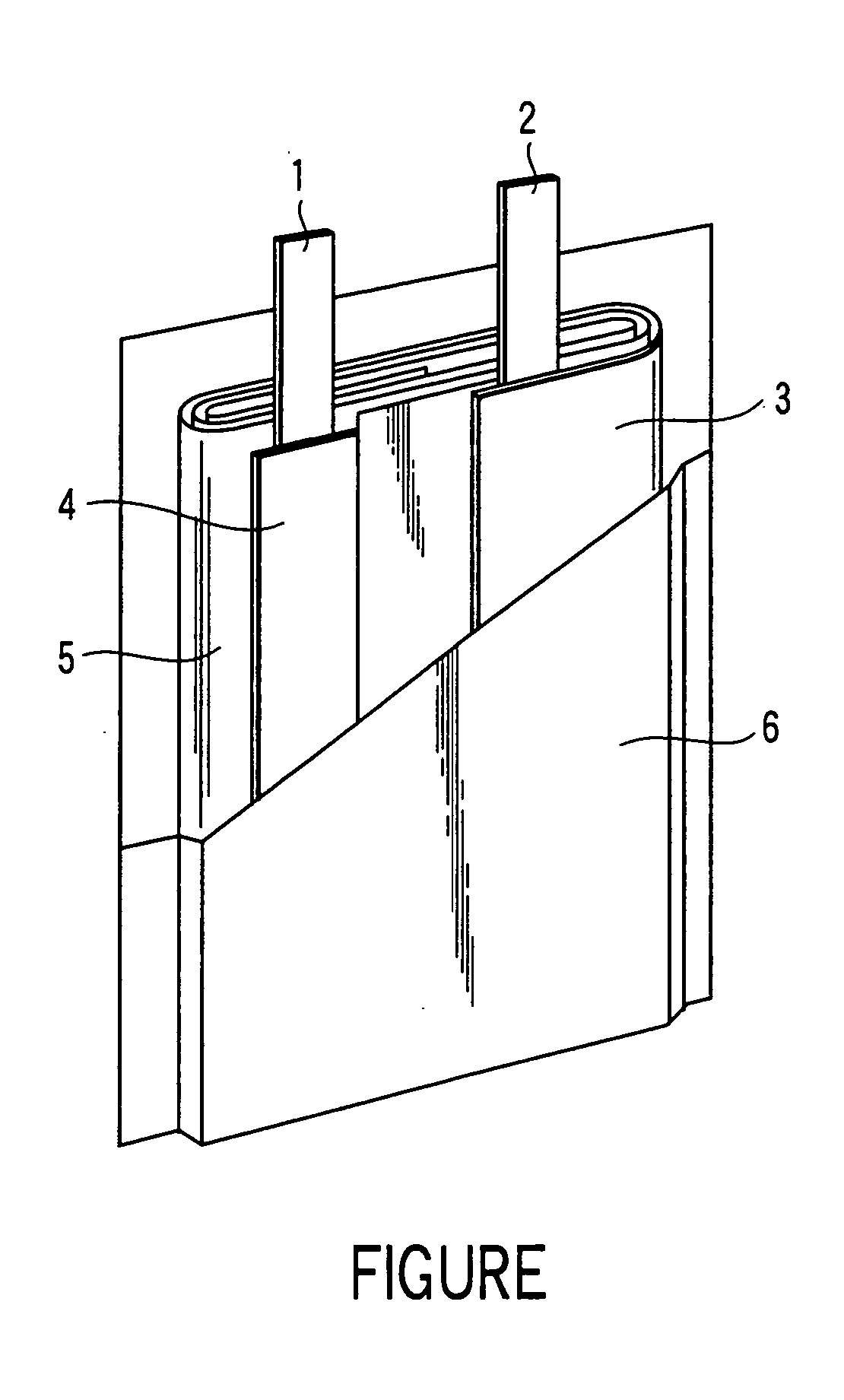
A battery having the structure shown in FIGURE 1 was fabricated. Examples 2 to 4 and Comparative examples 1 to 4 are same in structure as shown in FIGURE 1.
<Preparation of Positive Electrode>
By blending 90 wt. % of lithium cobalt oxide (LiCoO2) as an active material, 3 wt. % of acetylene black, 3 wt. % of graphite, and 4 wt. % of polyvinylidene fluoride (PVdF) in n-methyl pyrrolidone (NMP), a slurry was prepared. The slurry was applied on both sides of a current collector made of an aluminum foil of 15 μm in thickness, and dried and pressed, and a positive electrode with electrode density of 3.0 g / cm3 was prepared.
<Preparation of Negative Electrode>
By blending Li4Ti5O12 as a negative electrode active material, coke with average particle size of 1.12 μm and specific surface area of 82 m2 / g as an electronic conductor, and polyvinylidene fluoride (PVdF) by a ratio of 90:5:5 by weight, the mixture was dispersed in n-methyl pyrrolidone (NMP) solvent, and a slurry was prepa...
example 2
A nonaqueous electrolyte secondary battery same as in Example 1 was manufactured except that the electronic conductor was replaced by coke of which average particle size is 3.37 μm, specific surface area is 25.7 m2 / g, spacing (d002) of (002) plane is 0.3472 nm, and crystallite size (Lc) in the C-axis direction is 1.90 nm.
example 3
A nonaqueous electrolyte secondary battery same as in Example 1 was manufactured except that the electronic conductor was replaced by coke of which average particle size is 5.87 μm, specific surface area is 12.7 m2 / g, spacing (d002) of (002) plane is 0.3443 nm, and crystallite size (Lc) in the C-axis direction is 1.90 nm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com