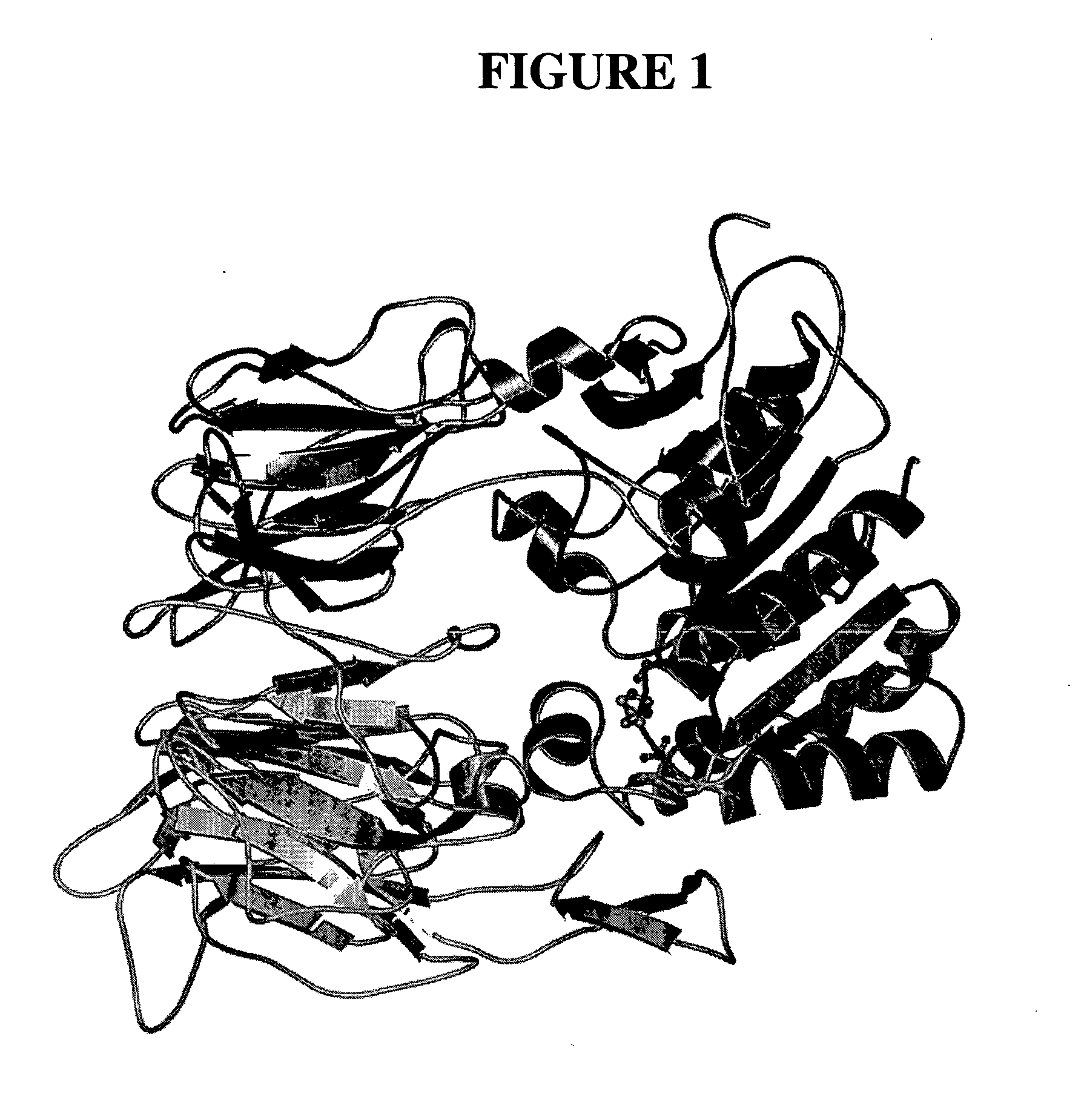
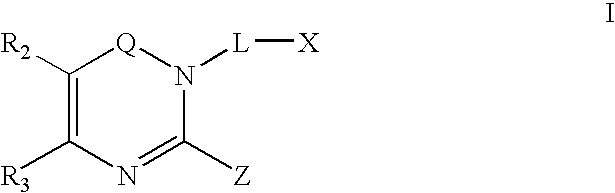Dipeptidyl peptidase inhibitors
a technology of peptide inhibitors and peptides, which is applied in the field of dipeptidyl peptidase inhibitors, can solve the problems of short half-life of glp-1 (7-36), infertility and amenorrhea, and rapid degradation of glp-1 in vivo
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1a
5-Bromo-2-chloro-3H-pyrimidin-4-one
5-Bromo-2,4-dichloro-pyrimidine (5.0 g, 22 mmol) was stirred in THF (10 mL) with 1N NaOH (30 mL) at r.t. for 3 h. The solution was made slightly acidic with 1N HCl and was extracted with CHCl3. Organics were dried (MgSO4) and concentrated in vacuo. Precipitation from 20% CHCl3 / hexanes and collection by filtration gave 2.92 g (64%) of 5-bromo-2-chloro-3H-pyrimidin-4-one as a white solid. 1H NMR (400 MHz, DMSO-d6): δ 13.33 (br s, 1H), 8.35 (s, 1H). MS (ES) [m+H] calc'd for C4H2N2OBrCl, 209, 211, 213; found 209, 211, 213.
example 1b
2-(5-Bromo-2-chloro-6-oxo-6H-pyrimidin-1-ylmethyl)-benzonitrile
5-Bromo-2-chloro-3H-pyrimidin-4-one (1.88 g, 9.0 mmol) was stirred in DME (25 mL DMF (5 mL) under nitrogen at 0° C. Sodium hydride (95%, 238 mg, 9.4 mmol) was added in portions. After 10 min, lithium bromide (1.56 g, 17.9 mmol) was added and the reaction stirred for 15 min at r.t. α-Bromo-o-tolunitrile (3.5 g, 17.9 mmol) was added, and the reaction stirred at 65° C. for 8 h. The solution was diluted with EtOAc, washed with brine, dried (MgSO4) and concentrated in vacuo. Purification by silica gel chromatography (1:1:1 EtOAc / hexanes / CHCl3) gave 997 mg (34%) of 2-(5-bromo-2-chloro-6-oxo-6H-pyrimidin-1-ylmethyl)-benzonitrile as a white solid. 1H NMR (400 MHz, CDCl3): δ 8.11 (s, 1H), 7.73 (dd, 1H, J=7.6, 1.2 Hz), 7.58 (dt, 1H, J=7.6, 1.2 Hz), 7.45 (t, 1H, J=7.6 Hz), 7.16 (d, 1H, J=7.6 Hz), 5.69 (s, 2H). MS (ES) [m+H] calc'd for C12H7N3OBrCl, 324, 326, 328; found 324, 326, 328.
Also obtained from the reaction were impure f...
example 1
2-[2-(3-(R)-Amino-piperidin-1-yl)-5-bromo-6-oxo-6H-pyrimidin-1-ylmethyl]-benzonitrile, TFA salt
2-(5-Bromo-2-chloro-6-oxo-6H-pyrimidin-1-ylmethyl)-benzonitrile (189 mg, 0.58 mmol), (R)-3-amino-piperidine, dihydrochloride (128 mg, 0.74 mmol) and sodium bicarbonate (195 mg, 2.32 mmol) were stirred in ethanol (5 mL) at 60° C. for 90 min. The reaction was diluted with EtOAc, washed with water and brine, dried (MgSO4), and concentrated in vacuo. Purification by silica gel chromatography (5% MeOH / CHCl3) gave 139 mg (62%) of the title compound as a clear oil. This was converted to the solid TFA salt by subjection to TFA in CH2Cl2 and concentration in vacuo. 1H NMR (400 MHz, DMSO-d6): δ 8.18 (s, 1H), 7.98 (br s, 3H), 7.82 (d, 1H, J=6.8 Hz), 7.64 (dt, 1H, J=7.6, 1.2 Hz), 7.47 (t, 1H, J=7.2 Hz), 7.27 (d, 1H, J=7.6Hz), 5.29 (AB q, 2H, J=42.8, 15.2 Hz), 3.52-3.57 (m, 1H), 3.30-3.39 (m, 1H), 3.15-3.24 (m, 1H), 2.88-3.05 (m, 2H), 1.90-1.99 (m, 1H), 1.75-1.83 (m, 1H), 1.49-1.63 (m, 2H). MS (ES) [...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| amino-acid | aaaaa | aaaaa |
| adhesion | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com