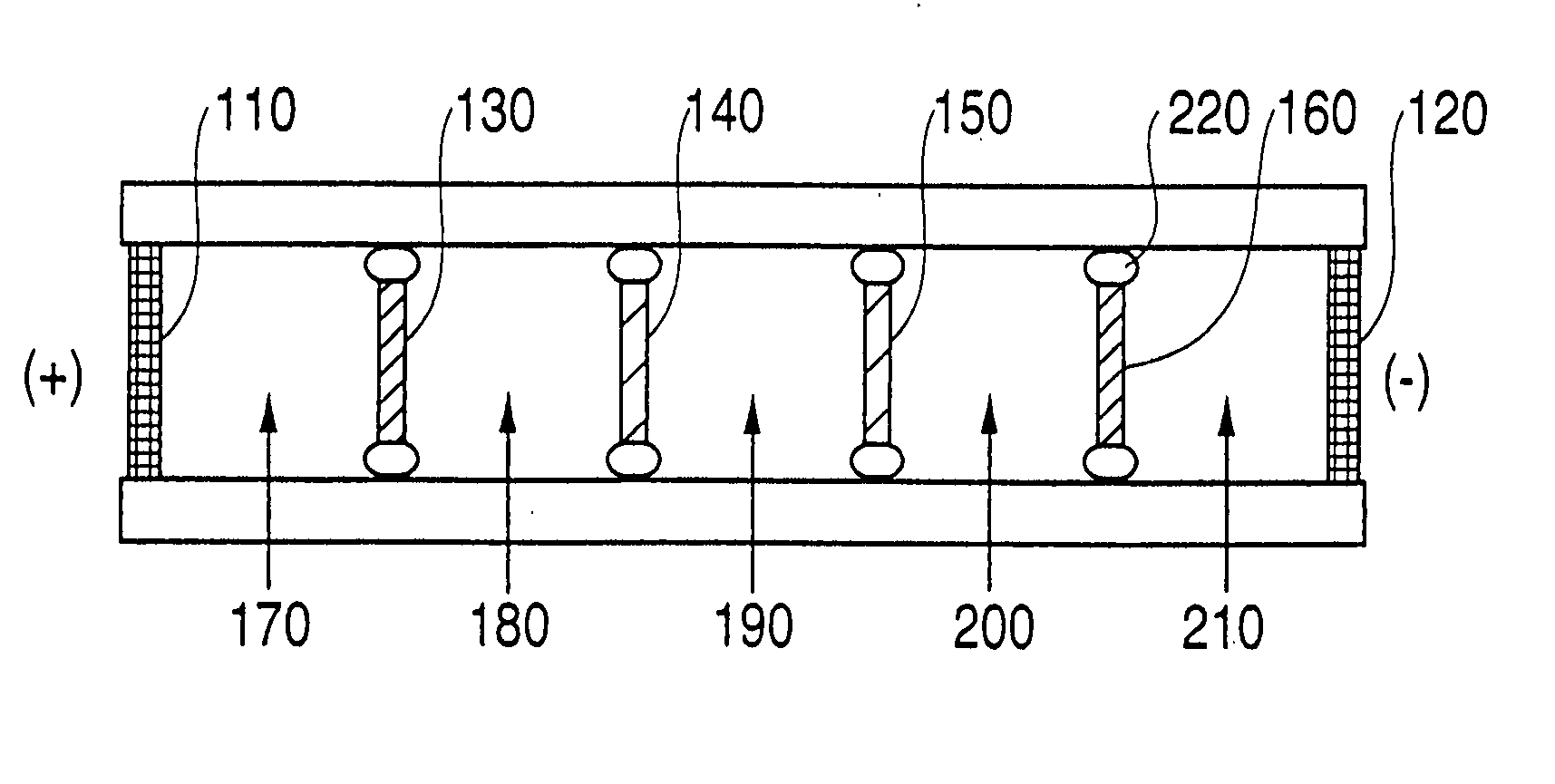
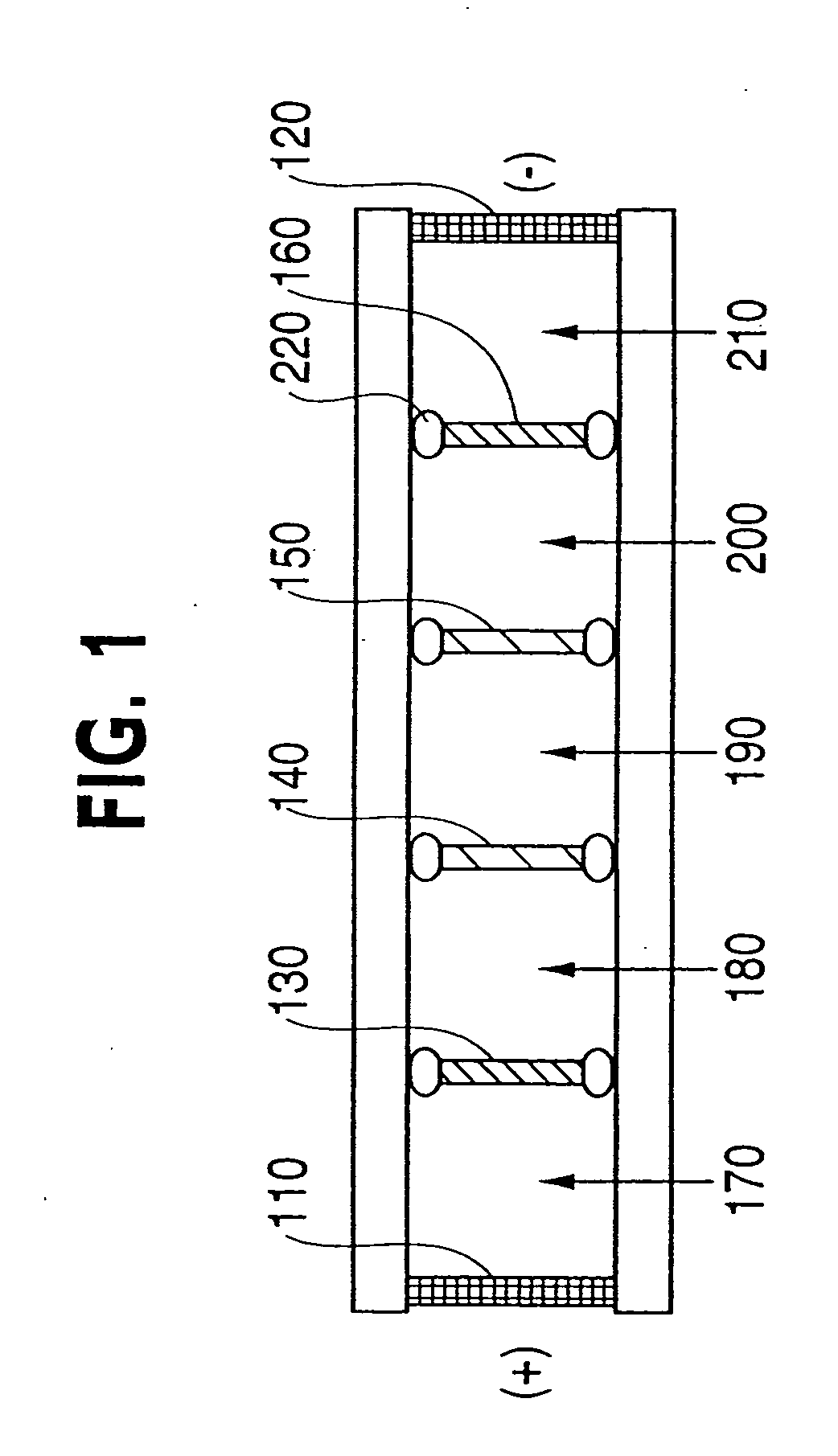
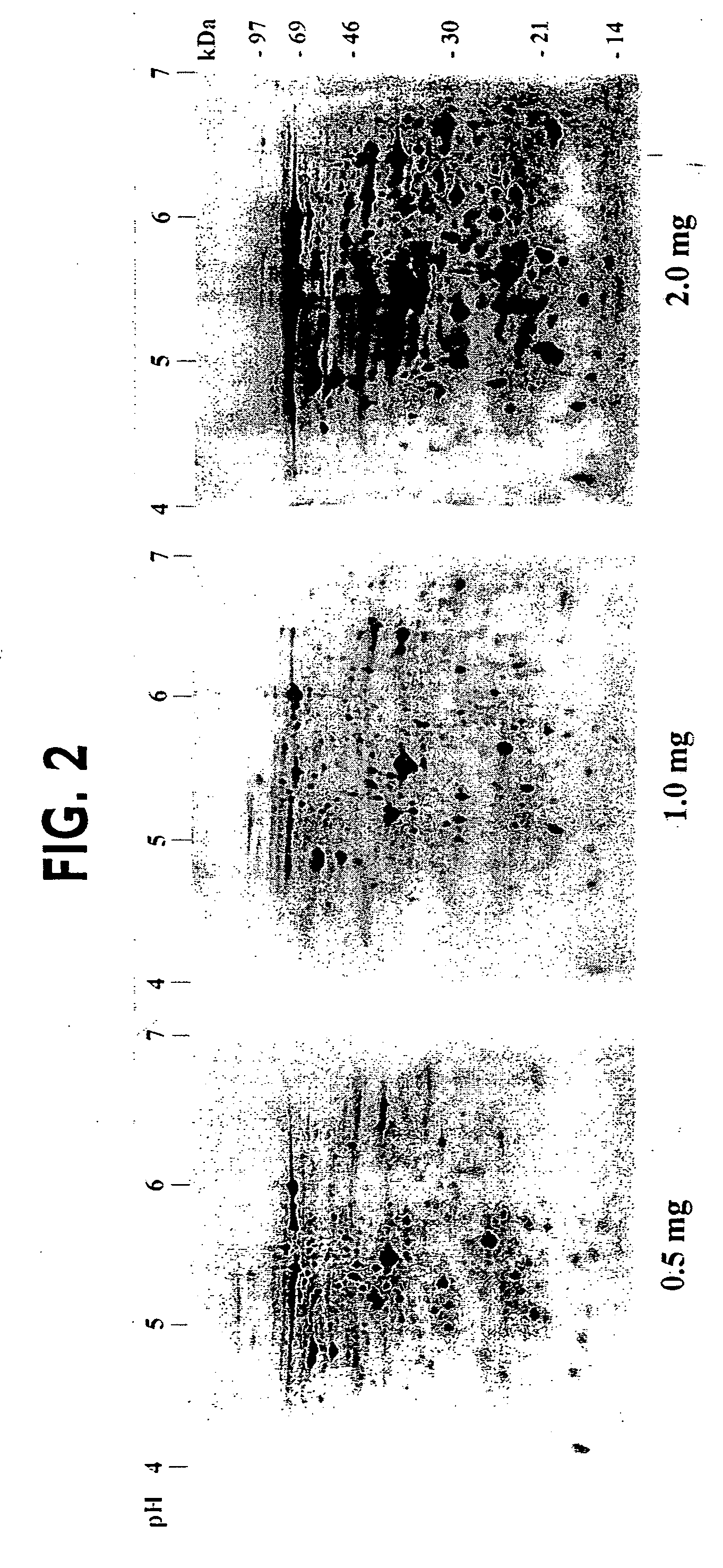
Method and device for separation of charged molecules by solution isoelectric focusing
a technology of isoelectric focusing and charged molecules, applied in the direction of fluid pressure measurement, liquid/fluent solid measurement, peptide measurement, etc., can solve the problems of low maximum sample loading capacity, lack of resolution and dynamic range for resolving and detecting large numbers of charged molecules, and high cost of separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Metabolically Radiolabeled Escherichia coli Extracts
[0054] Metabolically radiolabeled E. coli extracts were used in these studies to systematically evaluate protein recoveries. E. coli was selected since this relatively simple organism can be readily metabolically radiolabeled to high specific activity to provide sensitive and reliable detection of protein losses. In contrast to chemical labeling methods such as iodination of a portion of the sample, metabolic radiolabeling of the entire sample ensured that the labeling method would not alter the properties of the proteins and that each protein was a homogeneous population of molecules.
[0055]E. coli were cultured as previously described (Harper & Speicher, (1995) in CURRENT PROTOCOLS IN PROTEIN SCIENCE (Coligan et al., eds.), pp. 6.6.1-6.6.21, John Wiley & Sons Inc., New York) with modifications. Briefly, E. coli cells were inoculated in Luria broth (LB medium) and incubated at 37° C. with continuous shaking (250-30...
example 2
Two-Dimensional Electrophoresis
[0057] Isoelectric focusing equipment, IPG gels, and relevant reagents were purchased from Amersham Pharmacia Biotech (San Francisco, Calif., USA), unless otherwise indicated. Proteins were isofocused using different pH range IPG strips (pH 3-10 NL, 4-7L and 4.8-6.2L, 18 cm length) on the IPGphor™ Isoelectric Focusing System. Narrow pH range IPG gels (pH 4.8-6.2L) were cast in the laboratory using cornmercial immobilines as detailed in the IPG application manual. Immediately prior to isoelectric focusing, dried IPG strips were rehydrated for 8 h with sample in IPG sample buffer (350 μl) in the ceramic strip holders (1 h without current followed by 7 h at 30 Volts) as described by Gorg et al. (1999) Electrophoresis 20, 712-717. The IPG sample buffer contained 2 M thiourea, 7 M urea, 0.1 M DTT, 4% CHAPS and 2% IPG-buffer (carrier ampholyte mixture matching the pH range used). After the 8 h rehydration, samples were focused for 1 h each at 500 V, 1000 V,...
example 3
Determination of Protein Recoveries
[0059] Protein recoveries and losses throughout alternative prefractionation methods were determined using liquid scintillation counting. Any surfaces that contacted samples were extracted with 1% SDS to remove any adsorbed or precipitated proteins. Typically, a small volume of these SDS extracts or sample solutions (5 μl) was mixed with 4.5 ml of Bio-Safe II scintillation cocktail (Research Products International Corp, Mt. Prospect, Ill., USA) and radioactivity was counted using a Model 1500 TRI-CARB Liquid Scintillation Analyzer (PACKARD Instrument Company, Downers Grove, Ill., USA). The radioactivity left in gels after elution was counted after solubilization using 1 N NaOH at 60° C. for 3 h, followed by neutralization with concentrated HCl and addition of the scintillation cocktail.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com