Antibody formulation
a technology of antibody and formulation, which is applied in the direction of antibody medical ingredients, immunoglobulins, peptides, etc., can solve the problems of chemical instability, physical instability, special problems in the formulation of such proteins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
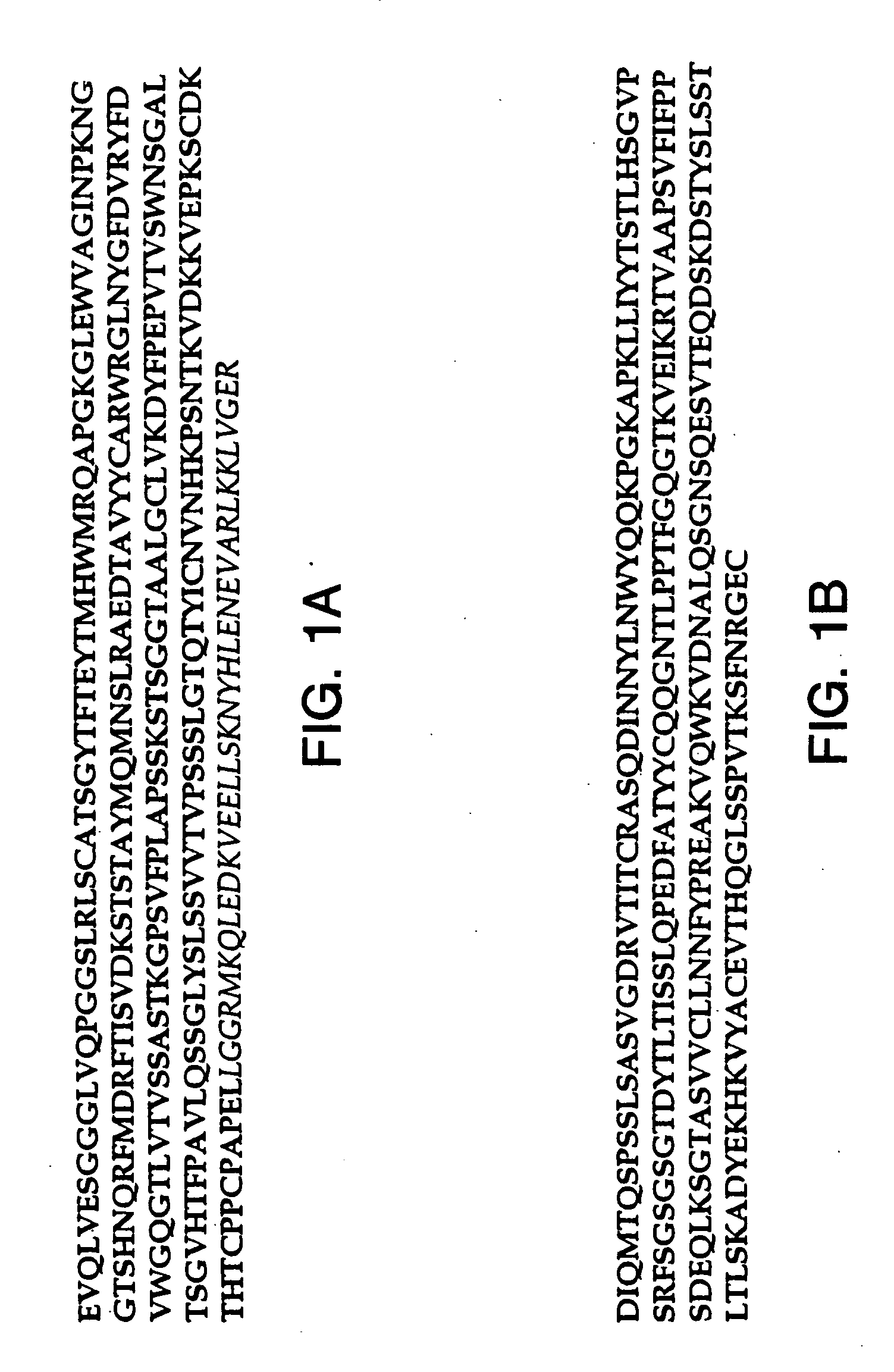
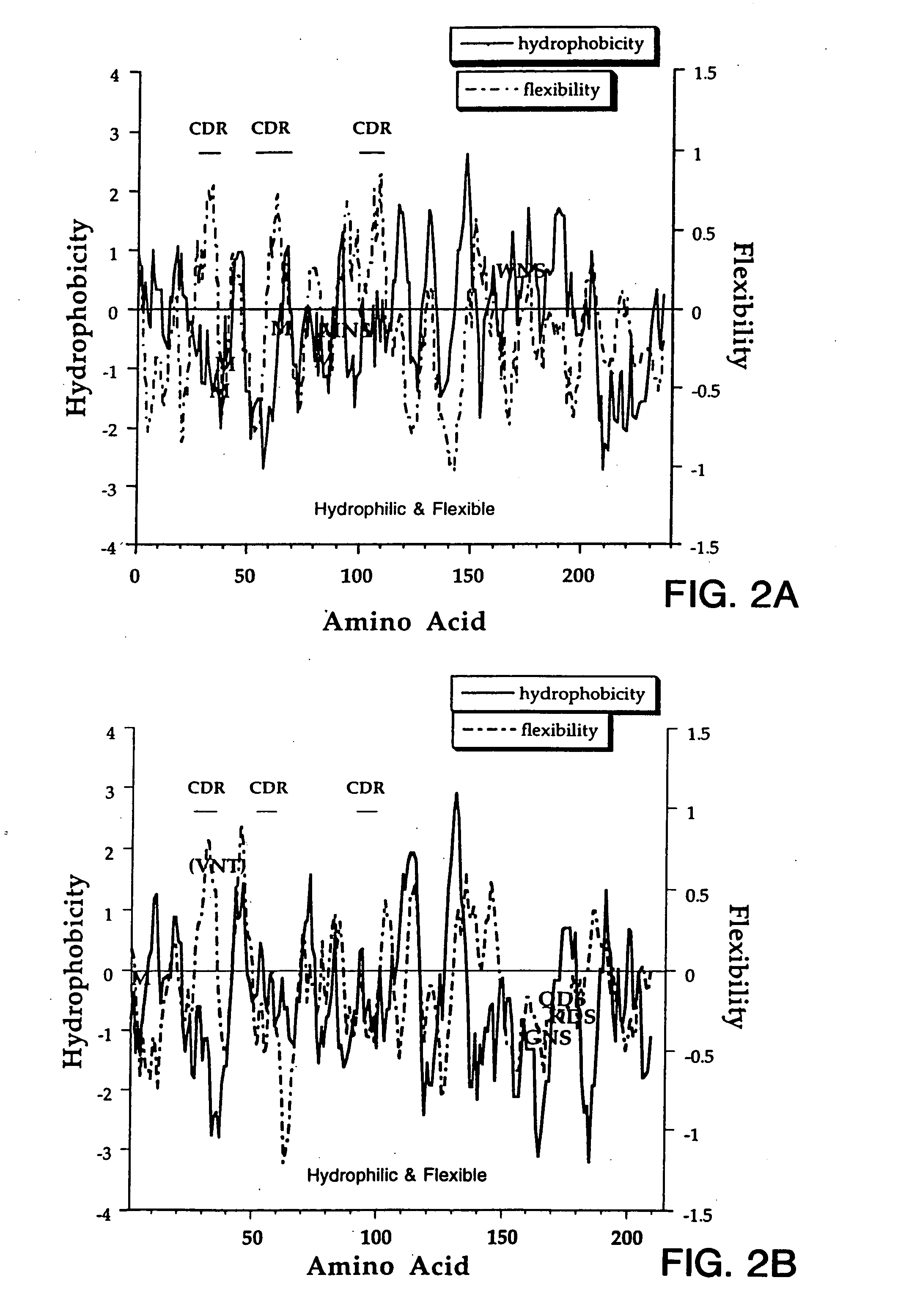
[0146] This example describes an aqueous formulation comprising the antibody, recombinant humanized anti-CD18 antibody (rhuMAb CD18). RhuMAb CD18 having the amino acid sequence shown in FIG. 1A (heavy chain; SEQ ID NO:1) and FIG. 1B (light chain; SEQ ID NO:2) was created by humanization of the murine monoclonal antibody muMAb H52 (Hildreth et al. J. Immunology 134:3272-3280 (1985)).
[0147] The rhuMAb CD18 was produced recombinantly as described below. Plasmid pS1130 was constructed to direct production of a rhuMAb CD18 precursor molecule with a leucine zipper domain in E. coli. The precursor is cleaved during the purification process by the protease pepsin to yield rhuMAb CD18. rhuMAb CD18 is an F(ab′)2 molecule composed of two different peptides (light and heavy chains) linked by disulfide bonds. Fusion of a yeast GCN4 leucine zipper dimerization domain to the C-terminus of an Fab′ substitutes for the Fc region and allows for efficient F(ab′)2 production in E. coli. The GCN4 leucin...
example 2
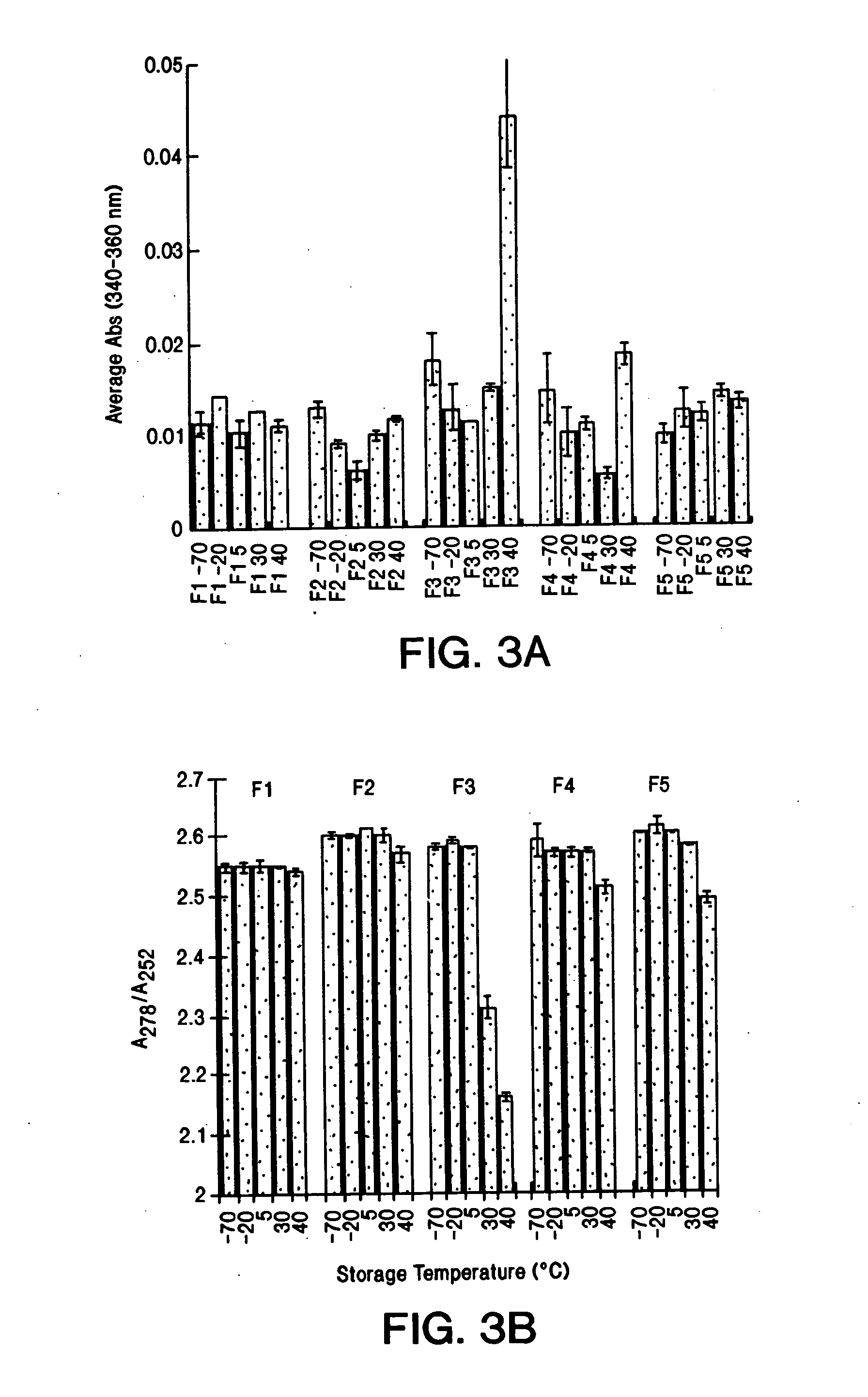
[0204] This example describes the production of a stable aqueous multidose formulation comprising a recombinant humanized anti-CD20 antibody, rhuMAb CD20. Acetate (pH 5) formulations stored at 40° C. for one month demonstrated greater stability than those samples formulated in histidine (pH 5 or 6). The histidine formulations after accelerated temperature storage became very opalescent and yellow in color. A buffering capacity of 10-30 mM acetate was sufficient to maintain the pH at 5.0. The effective amount of tonicity modifier needed to stabilize the antibody against freeze or thermal induced aggregation was compared using sodium chloride (NaCl) ortrehalose. Trehalose was found to protect the formulation from freeze induced aggregation, particularly at levels ≧134 mM (500:1 molar ratio). The trehalose formulations (67-270 mM) were much more effective than NaCl in stabilizing formulations placed at 40° C. as evidenced by the clarity of the solution. These results led to the develop...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com