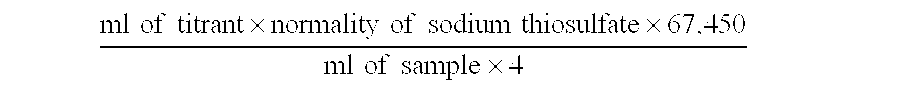Methods for making chlorous acid and chlorine dioxide
a chlorous acid and chlorine dioxide technology, applied in the direction of organic compounds/hydrides/coordination complex catalysts, physical/chemical process catalysts, halogen oxides/oxyacids, etc., can solve the problems of slow decomposition, difficult control of reaction, and inability to complete reaction after 60 minutes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
specific embodiments and examples
DESCRIPTION OF SPECIFIC EMBODIMENTS AND EXAMPLES
Precursor Solution for Examples 1-6
[0045] In Examples 1-6, a single chlorite precursor solution was used for all Examples. The chlorite precursor solution was prepared by diluting an aqueous 25% sodium chlorite solution with reverse osmosis water. The pH of the resultant solution was measured to be 8.5 with a Hach Sension 1 pH meter. The chlorite concentration in the precursor solution was measured to be 823 mg / L by using a Hach Digital Titrator, Iodometric Test Kit for Chlorine. To begin the measurement, 100 ml of reverse osmosis water was placed in a 250-ml Erlenmeyer flask, and 2 ml of the sample precursor solution was placed into the reverse osmosis water. One Potassium Iodide Powder Pillow and one Dissolved Oxygen Reagent 3 Pillow were added to the solution in the flask, swirled to mix, and placed in the dark for 10 minutes to allow the reaction to go to completion. Using a 0.113 N Sodium Thiosulfate Cartridge in the Digital Titr...
example 1
Chlorous Acid Generation by Cation Exchange
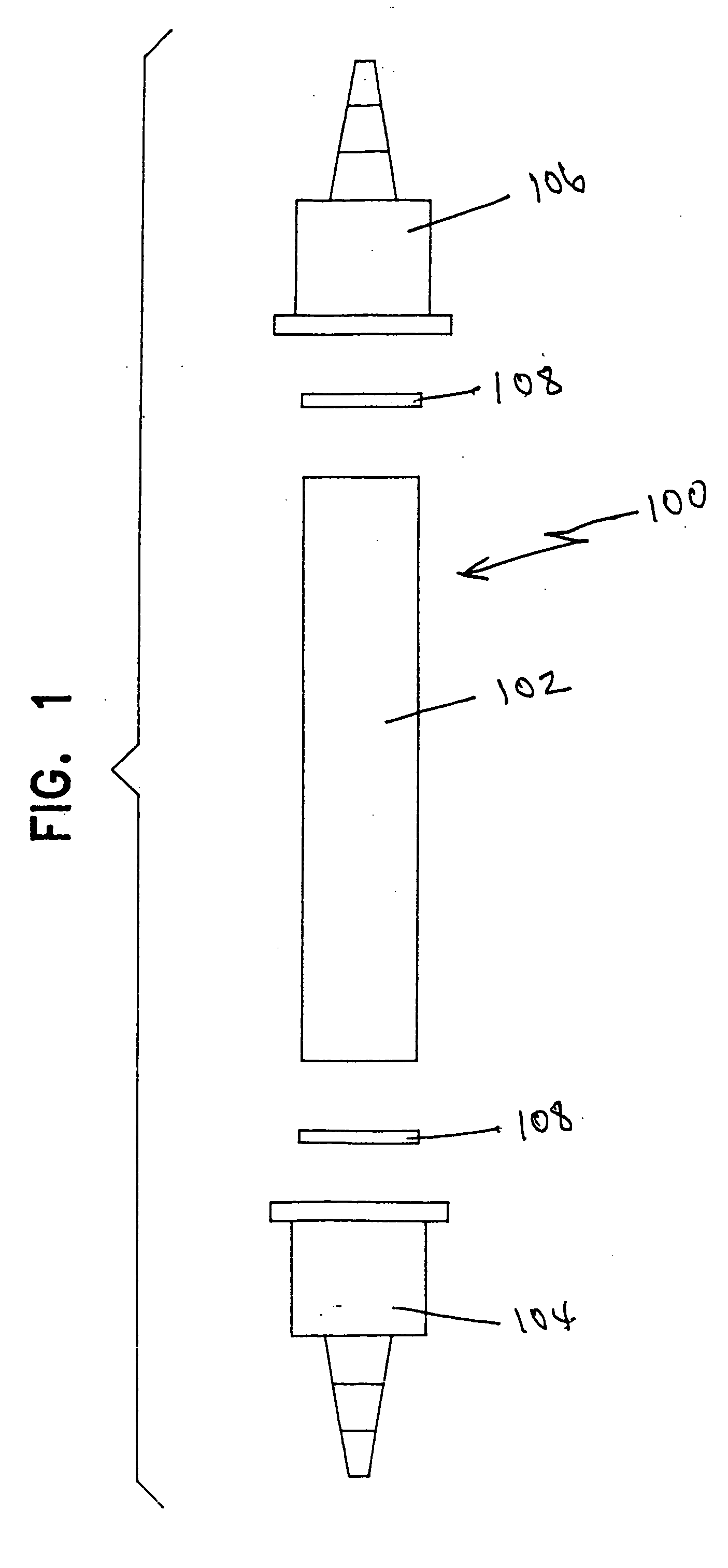
[0047] In Example 1, one 30 ml plastic test tube 100 as shown in FIG. 1 was clipped to a wall with pipe clips. The feed tubing ran from a reservoir containing the precursor solution to the bottom of the tube. The product tubing ran from the top of the tube to a brown sample bottle. In this example, the tube was filled with a commercially available strong acid organic cation resin in the hydrogen form, sold under the name Resintech CG-8, such that the tube was full.
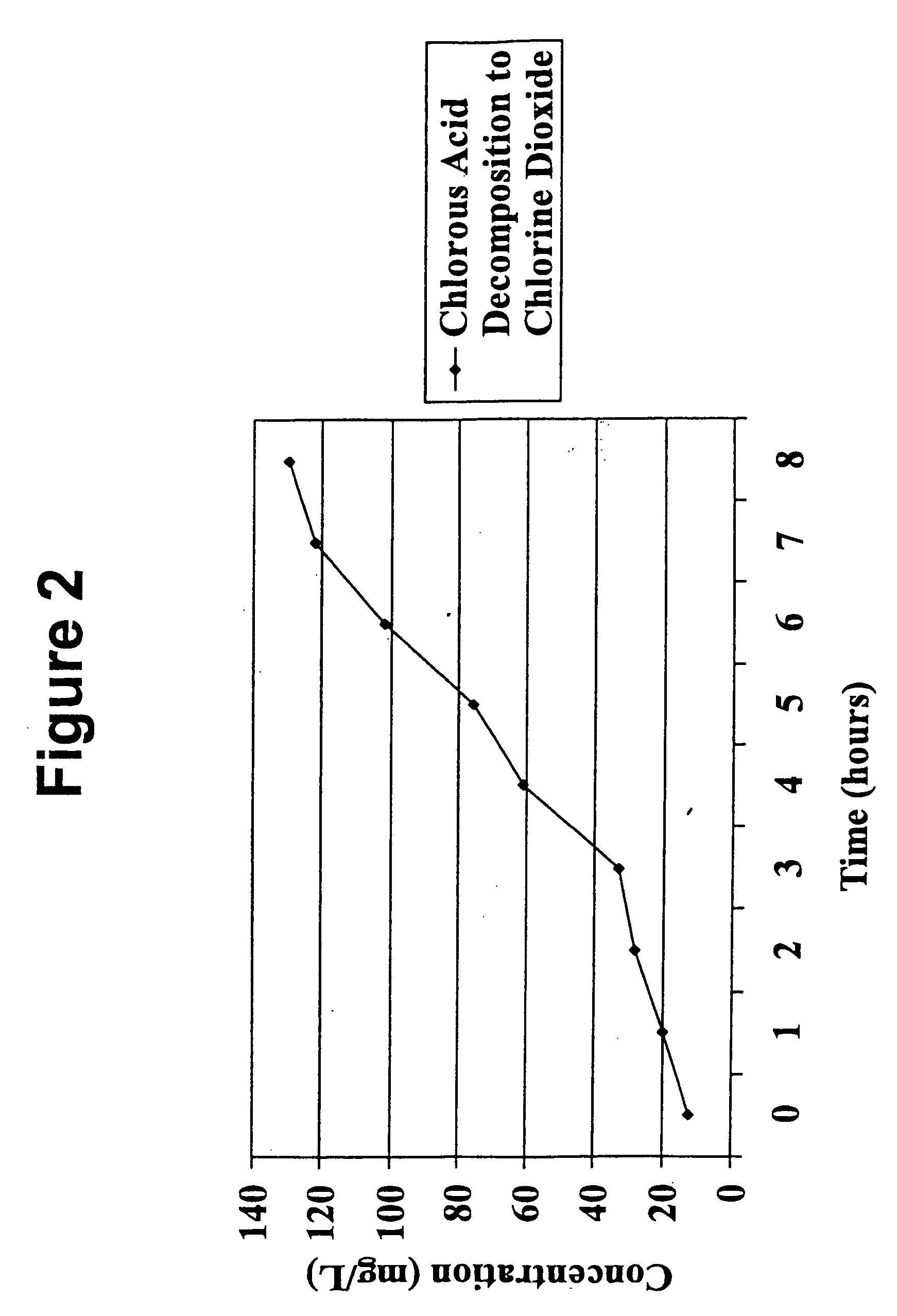
[0048] A continuous stream of the chlorite precursor solution was passed upwardly through the tube such that the flow rate was 30 ml / min. A 250 ml sample of solution was taken from the tube's top end and placed in the brown bottle, sealed, and stored in a dark cabinet. A Hach 2010 Spectrophotometer using Method 8138 for the measurement of chlorine dioxide (0-700 mg / L) was used to test the stored sample for chlorine dioxide at one-hour intervals for eight hours.
[0049] The result...
example 2
Chlorous Acid Generation by Cation Exchange from a Chlorite Precursor and Subsequent Catalytic Chlorine Dioxide Generation
[0050] In Example 2, two identical 30 ml plastic test tubes 100 as shown in FIG. 1 were clipped to a wall with pipe clips. Interconnecting plastic tubing ran from the first test tube to the second so that solution flowed from the bottom to the top of each test tube. The feed tubing ran from a reservoir containing the precursor solution to the bottom of the first test tube. The product tubing ran from the top of the second test tube to the flow-through cell of a Hach 2010 Spectrophotometer using Method 8138 for the measurement of chlorine dioxide (0-700 mg / L).
[0051] (A) The first test tube was filled with the Resintech CG-8 strong acid organic cation resin in the hydrogen form such that the tube was full. The second test tube was packed with a commercially available inorganic cation resin in the hydrogen form, sold under the name Resintech SIR-600, having platin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentrations | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| flow rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com